
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
CLINICAL STUDIES / ETUDES CLINIQUES
PREDICTIVE VALIDITY AND USEFULNESS OF VISUAL SCANNING TASK IN HIV/AIDS – A CASE CONTROL ANALYSIS
VALIDITÉ PRÉDICTIVE ET UTILITÉ D'UN TEST NUMÉRIQUE DE CHARGE DE LECTURE LORS DE VIH/SIDA. UNE ANALYSE CONTROLÉ
E-Mail Contact - OGUNRIN Olubunmi :
bfunmi@uniben.edu
ABSTRACT Background Objective Methods Results Key words: brain, cognition, HIV/AIDS, predictive validity, visual scanning test RESUME Introduction Objectif Méthodes Résultats Mots clés: Cognition, VIH, Neuropsychologie, test visuel, SIDA INTRODUCTION The HIV/AIDS pandemic has become a global health concern. Its impact on human sufferings, cultures, demographics, economics and even politics is enormous. The sub-Saharan Africa and the Asian countries have been worst hit with increasing mortality, decreasing fertility and depletion of the productive sector of the population20. The prevalence of HIV/AIDS in Nigeria was 5.8% (adult population) as at the end of 200323. Ninety percent of 36.1 million HIV/AIDS patients live in developing countries and 25.3 million are in sub-Saharan Africa with an adult prevalence of 8.8%18,22. There are evidences to show that human immunodeficiency virus (HIV) damages the brain directly through the elaboration of virally mediated toxins (gp120, tat and nef) and indirectly through host toxins (quinolinic acid, tumour necrosis factor alpha, platelet activating factor (PAF), nitric oxide (NO) and neopterins)2,14,21. These neurotoxic effects promote neurodegeneration by synergism of HIV products with endogenous excitotoxins and interference of HIV with effect of growth factors that are essential for neuronal survival and maintenance. Several studies have demonstrated early brain changes using neuro-imaging techniques (computerized axial tomography and/or magnetic resonance imaging), and functional imaging studies to demonstrate cerebral metabolic dysfunction both at regional and cellular levels5,6,7. The consequences include neuro-cognitive impairments, which manifest either as minor cognitive motor disorder (MCMD) or HIV-associated dementia (HAD). These neuro-imaging facilities are not available in most sub-Saharan African countries. Neurocognitive impairments in the presence of brain damage have been detected with the computerized visual scanning task (CVST) invented by Goldstein et al4. We used the visual scanning task as a tool to assess the complex information processing and perceptual mental strategies in Nigerian patients with HIV/AIDS and inferred the presence or absence of brain damage based on their performances as compared with healthy, HIV-negative controls. SUBJECTS AND METHODS A total of 192 subjects out of 720 patients with positive ELISA test to HIV were randomly selected (using the table of random numbers) from the HIV/AIDS clinic of the University Teaching Hospital, Benin City, Nigeria over a 6 month period (January – June 2004). Then 96 controls were selected randomly from hospital staff members of the outpatient and antenatal clinics. A total of 288 subjects were recruited for the study. The subjects comprised 3 categories: 96 HIV-positive asymptomatic subjects, 96 symptomatic AIDS subjects and 96 healthy sero-negative controls. The ELISA method was used to detect HIV antibodies and diagnose HIV infection, and the CD4 levels were obtained for all subjects with the use of an automated flow cytometry (CyFlow; Partec GmbH, Munster, Germany) and double-checked with the manual CD4 kit (Coulter®; Partec GmbH, Munster, Germany). All the subjects were matched for age, sex and level of education. Informed consents were obtained from the patients and controls, and approval to undertake study was granted by the Hospital Ethics Committee. All subjects were interviewed using a basic questionnaire by one of the authors (OF) to obtain demographic variables. The exclusion criteria included subjects less than 18 years of age, patients already on anti-retroviral therapy, those with opportunistic infections (OIs), and patients with co-morbidity (diabetes mellitus, hypertension epilepsy, and associated intracranial disorders e.g. brain tumor, and other metabolic diseases), those with inconclusive diagnosis, major axis 1 psychiatric illness, presence of clinical signs of cardiac failure, alcohol intake above 120gm/week or 13units/week, history of previous head injury with loss of consciousness and patients on anti- cholinergic medications. COGNITIVE ASSESSMENT TOOL The computer-assisted visual scanning task (CVST) was used for cognitive assessment. This test was described by Goldstein et al4 in 1973 as a successful instrument for discriminating brain damage from normal and psychiatric populations. DeMita & Johnson developed a computerized version of the same task for the DEC LSI-11/03 computer3. The task has been adapted for the microcomputer by Moerland et al13 as part of the test battery, the Iron Psychology (acronym FePsy’). The task consists of finding a grid pattern out of 24 which matches the one in the centre of the screen (Fig 1). Grid patterns are displayed in a checkerboard fashion and are numbered from 1 to 24. The target pattern is marked by an arrow on the right side and is selected by typing the correct number from the keyboard. 24 different patterns are presented. After 12 presentations the surrounding grids change. Each subject had a total of 24 trials. The testee is asked to react as fast as possible. Results show accuracy and speed of responses and are evaluated within the context of visual (complex) information processing and perceptual-mental strategies. An important variable appears to be the average response time. The average scores were 15.8 seconds (range 7-18 seconds) for a normal control group4,13. Test presentation and response registration were controlled by a microcomputer, but one of the authors (AOO) was always present to adjust instructions to the individual performance level of the patients. The cognitive assessment was blind as the author (AOO) was not aware of the patients’ HIV status. It is pertinent to mention that the subjects need not be computer literate to perform the test, as they only need to carry out instructions as they relate to the test. The test is not level of education-based as the tests for primary do not differ from secondary and tertiary levels of education. Language does not affect performance. The test was administered in a reasonably quiet, well-lit room at room temperature of between 22oC and 25oC, between the hours of 10:00 and 13:00 each day of testing with the subject sitting comfortably at a distance of 40 to 60 centimeters from the visual display screen. Care was taken to ensure good brightness and contrast of screen and sufficient sound level of the speaker. STATISTICAL ANALYSIS The means of the ages and CD4 levels of the three categories were compared using two-way ANOVA. The means of the response times of the asymptomatic and symptomatic HIV-positive subjects were independently compared with the mean response time of the controls. The level of significance was determined using the chi square for trend and taken as p<0.05. A 2X2 contingency table was used to determine the predictive validity of the visual scanning task using the sensitivity, specificity and predictive values. RESULTS The mean ages were 32.9+ 8.0 years, 31.5 + 6.7 years and 33.6+7.1 years for the controls, asymptomatic HIV positive and symptomatic AIDS subjects respectively (p = 0.13). The means of the CD4 counts for the controls, HIV-positive asymptomatic and symptomatic subjects were 682±44, 284±62 and 142±36 respectively (p<0.05). The details of demographic information are presented in Table I. The mean response time for the controls, asymptomatic HIV-positive and symptomatic AIDS patients are presented in Table II. There was significant difference in the mean response times of the patients compared to the controls implying impaired perceptual mental functioning and visual information processing in the HIV-positive patients (p<0.05). The response time was prolonged in 31.6% of patients with CD4 levels > 500 (p>0.05), in 77.1% of those with CD4 levels between 200 and 499 and 86.7% of those with CD4 levels <200 (p<0.01). This observation revealed the role of severity of disease and degree of immuno-suppression on visual scanning performance of the affected patients implying that the more severe the disease the worse the cognitive impairment. An inverse relationship thus exists between CD4 levels and mean response times of the subjects - as the CD4 declines the mean response time increases (Fig 2). Abnormal or prolonged response time was observed in 18.4% (18 of 96) of the controls while 81.9% (157 of 192) of the HIV-positive subjects had abnormally prolonged response time. Using the 2X2 contingency table, the CVST has a sensitivity of 81.77%, specificity of 81.25%, predictive value positive (PVP) of 89.71%, predictive value negative (PVN) of 69.03% and an accuracy of 81.6%. DISCUSSION The predictive validity refers to how well a test will predict a subject’s performance with respect to some meaningful external criterion i.e. a real life event25. For example, a good performance on tests of visual or verbal memory is a good predictor that the subject has difficulty remembering things in day-to-day life. So for a clinician, the issue of validity of an instrument is whether the test is predictive with respect to an external criterion that is clinically meaningful. Predictive validity is better understood, in the clinical setting, in terms of specificity and sensitivity. In other words, the predictive value of a medical test is measured not by correlational analysis, as in psychometrics, but by conditional probability. It is based on this assertion that we chose to assess the predictive validity of this test. A significant limitation of this study is our inability to correlate the patients’ cognitive performances with neuro-imaging changes (CT or MRI). The prohibitive costs of these facilities in Nigerian hospitals, ranging from 33,000naira (~$254) to 60,000naira (~$462) for CT and 70,000naira (~$538.5) to 120,000naira (~$924) for MRI, militated against this. The pathogenesis of cognitive dysfunction, including impaired complex visual processing and perceptual mental strategies, in HIV/AIDS has been linked to structural and metabolic changes induced by the virus in the brain[8,14,17]. Cerebral atrophy, often accompanied by ventricular enlargement, represents the most common structural changes seen in either computerized axial tomography (CT) or magnetic resonance imaging (MRI)[5,6]. Focal areas of reduced cerebral perfusion were demonstrated by single photon emission tomography in patients with HIV-associated dementia (HAD) as well as in asymptomatic subjects with CD4 counts greater than 500/µl suggesting that brain damage appear during the early stages of infection[19]. Furthermore magnetic resonance spectroscopy (MRS) has shown a reduction in N-acetyl-aspartate, a marker of mature neurons in patients with advanced dementia16 and, in addition, significant elevations in brain choline and myoinositol have been found in all stages of HIV infection providing a marker of early brain involvement[10]. Positron emission tomography with fluoro-deoxygenase has shown two general patterns of metabolic alterations in the HIV brain, hypermetabolism in the basal ganglia and thalamus, possibly characteristic of early stages of HAD and hypometabolism in the cortex that probably correlates with the severity of dementia[5]. The pattern of neurocognitive impairments in HIV infection resembles that seen in other sub-cortical frontal disorders like Parkinson’s disease[9]. There is agreement on the presence of cognitive impairments in symptomatic HIV-positive subjects but there are conflicting reports on its presence in asymptomatic HIV-positive individuals[1,12]. The cognitive decline may be subtle and may not be detectable by conventional neuropsychological tests however definite cognitive decline has been conclusively shown with severe immunosuppression[15]. It may also be that the HIV-induced brain changes are not severe enough to cause significant cognitive dysfunction in the asymptomatic stage. However the use of computerized cognitive testing has proved useful in identifying these subtle cognitive deficits[11,24]. CONCLUSION We recommend the use of the computerized visual scanning task, especially in clinical settings where functional neuro-imaging facilities are lacking, for the screening of significant brain damage in HIV/AIDS causing neuro-cognitive impairments. There is need for a study that will correlate cognitive impairments with functional neuro-imaging patterns among HIV/AIDS patients in sub-Saharan Africa.
(a) the asymptomatic HIV-positive subjects were independently compared with the controls 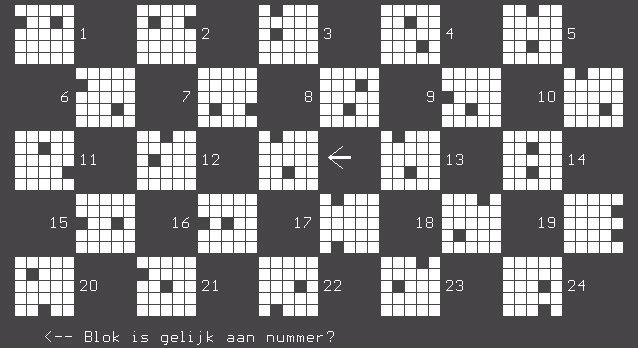 Fig 1 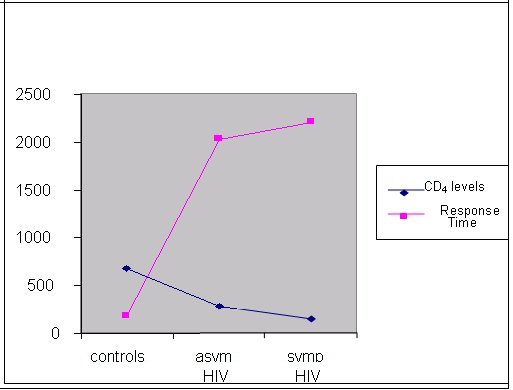 Fig 2 REFERENCES
|
© 2002-2018 African Journal of Neurological Sciences.
All rights reserved. Terms of use.
Tous droits réservés. Termes d'Utilisation.
ISSN: 1992-2647