CLINICAL STUDIES / ETUDES CLINIQUES
OUTCOME AFTER ACUTE TRAUMATIC SUBDURAL HAEMATOMA IN KENYA: A SINGLE-CENTRE EXPERIENCE
EVOLUTION DES HÉMATOMES SOUS-DURAUX AIGUS POST-TRAUMATIQUES AU KENYA: EXPÉRIENCE D'UN CENTRE KÉNYAN
- Division of Neurosurgery, Department of Surgery, Kenyatta National Hospital, University of Nairobi, Nairobi, Kenya
- Department of Human Anatomy, School of Medicine, University of Nairobi, Kenya
E-Mail Contact - KIBOI Julius Githinji :
ABSTRACT
Background
Acute subdural haematoma (ASDH) is one of the most common traumatic neurosurgical emergencies with a high mortality rate. However, few studies have examined prognostic factors of outcome in isolated traumatic ASDH.
Methods
We reviewed the records of patients who were diagnosed with traumatic ASDH between January 2000 and December 2009. Analysis was carried out using Statistical Package for Social Sciences (SPSS) version 11.5 and multivariate logistic regression analysis used to evaluate the influence of clinical variables on outcome.
Results
A total of 259 patients were diagnosed with acute subdural hematomas during the study period. The mean age was 41.1 years + 19.659 and 223 (86.1%) were men while 36 (13.9%) were women. The most common cause of injury was assault (44.8%) with road traffic and falls accounting for 24.7% and 30.5%. Fifty two patients (20.1%) died while hospitalized while good functional recovery was attained by 118 (45.6%). Patients aged older than 61 years had a significantly higher mortality rate (30.6%) and a lower rate of good functional recovery (24.5%) (P=0.073). Of the patients with GCS scores <8, 38 (65.5%) died as compared to 4 (3.5%) deaths in patients with scores ranging from 13 to 15. Further, a history of loss of consciousness and the length of time between the injury and operative decompression significantly influenced the final outcome.
Conclusion
An increased risk of death occurs in patients who are over 61 years of age and have lower preoperative GCS, the presence of pupillary abnormalities and a long interval between trauma and decompression. The findings would help clinicians determine management criteria and improve survival.
Key Words: Acute subdural hematoma, Head injury, Functional recovery, Mortality.
RESUME
Fond
L’hématome sous dural aigu post-traumatique (HSDA) est une urgence neurochirurgicale avec un haut taux de mortalité. Peu d’études ont examiné les facteurs prognostiques lors d’ ASDH traumatisant isolé.
Méthodes
Nous avons réexaminé les dossiers de patients avec ASDH post traumatique entre janvier 2000 et décembre 2009. L’analyse a été pratiqué en utilisant le Packet Statistique pour les Sciences Sociales (SPSS) la version 11,5.
Résultats
Un total de 259 malades a été diagnostiqué avec les hématomes de subdural aigu pendant la période d’étude. L’âge moyen était 41,1 ans + 19,659 et 223 (86.1%) étaient des hommes pendant que 36 (13.9%) étaient des femmes. La cause la plus commune de blessure était l’assaut (44.8%) avec la circulation de route et les chutes représente 24,7% et 30,5%. Cinquante deux malades (20.1%) est mort pendant qu’a hospitalisé pendant que le bon rétablissement fonctionnel a été atteint par 118 (45.6%). Les malades plus vieux que 61 ans ont eu un significativement plus haut taux de mortalité (30.6%) et un taux plus bas de bon rétablissement fonctionnel (24.5%) (p=0.073). Des malades avec GCS scores <8, 38 (65.5%) est mort comme en comparaison de 4 (3.5%) les morts dans les malades avec scores étendant de 13 à 15. Plus, une histoire de perte de conscience et le temps qu'il faut entre la blessure et la décompression opérative a influencé significativement l'issue finale.
Conclusion
Un risque augmenté de mort arrive dans les malades qui sont plus de 61 ans majeurs et a GCS préopératoire plus bas, la présence d’anomalies de pupillary et un intervalle long entre le traumatisme et la décompression. Les conclusions aideraient des practiciens déterminent les critères de direction et améliorent la survie.
INTRODUCTION
Traumatic acute subdural hematoma (ASDH) is one of the most common clinical entities encountered by any neurosurgical service and often requires surgical intervention (1, 2). ASDH occurs in 12% to 30% of patients with severe head injury (3) and reported mortality rates vary from 36% to 79% for patients who underwent surgery (4, 5). Although developments and improvements in emergency medical service systems, neuro-intensive monitoring and treatment, ASDH is a disorder with a very high mortality rate and extremely poor prognosis among traumatic brain injuries (5, 6, 7).
Therefore, identifying reliable prognostic factors for ASDH to improve the surgical results in these patients is important. However relatively few studies have focused on the factors that affect the outcome of patients with traumatic ASDH. Kenyatta National Hospital, located in Nairobi Kenya, is a national teaching and referral hospital with a bed capacity of 2000 and the largest neurosurgical unit in the Great Lakes region. Through this center, the great majority of neurosurgical referrals are managed. Among these patients, we retrospectively reviewed patients who were diagnosed and treated for traumatic ASDH and sought to identify the factors related to functional recovery and mortality of this lethal disorder with a secondary aim to improve functional outcome of these patients hereafter.
METHODOLOGY
Following ethical approval, the records of 259 patients who were admitted to the neurosurgical unit at the Kenyatta National Hospital and diagnosed with ASDH between January 2000 and December 2009 were reviewed. The records were analysed for demographic characteristics such as gender, age, GCS on admission, pupil abnormalities, mechanism of injury, and time elapsed from accident to surgery.
The GCS score is usually determined on admission and all patients were divided patients into three groups; those with GCS scores of 3 to 8, 9 to 12 and 13 to 15 for statistical analysis. For logistic regression analysis, patients were classified as having 0, 1, or 2 reactive pupils. Outcome was assessed according to the Glasgow Outcome Scale (GOS) at the time of discharge from hospital or mortality.
Data was collected in pre-formed questionnaires, coded and analysis carried out using Statistical Package for Social Sciences (SPSS) version 11.5. Frequencies and means were computed for description of the various variables, discrete variables compared using the Chi-square test and continuous variables compared using the Students’ t-test. A linear logistic multivariable regression model was run to determine which variables are independently associated with functional recovery and mortality. A P value <0.005 was considered statistically significant.
RESULTS
A total of 259 patients were diagnosed with acute subdural hematomas at the Kenyatta National Hospital during the study period. The mean age was 41.1 years + 19.659 with a range from 2 to 96 years; 223 (86.1%) were men and 36 (13.9%) were women. Majority (44.8%) of the patients were aged between 26 and 45 years while 3.9% and 17.8% were aged below 13 years and older than 61 years respectively. The most common cause of injury was assault (44.8%) with road traffic and falls accounting for 24.7% and 30.5% respectively.
(See Figure 1)
(See Figure 2)
(See Figure 3)
(See Figure 4)
Fifty two patients died while hospitalized and the overall mortality rate was 20.1%. Good functional recovery was attained by 118 (45.6%) while 26.6% and 6.6% of the patients had moderate and severe disability respectively. Twenty one percent of male patients died while hospitalized as compared to 13.9% female patients. In addition, 46.2% male patients achieved functional recovery as compared to 41.7% female patients. However, this relation of patients’ sex to outcome was not statistically significant (P=0.111).
A significantly higher mortality rate (30.6%) was observed for patients older than 61 years (P=0.003), while patients aged 26 to 45 and less than 13 years had a mortality of 23.7% and 20 % respectively. Furthermore, fewer (24.5%) patients aged >61 years had good functional recovery as compared to 50% and 57.4% patients aged <13 and 14-25 years respectively (P=0.003). In addition, the preoperative GCS score was highly correlated with outcome. Of the 58 patients with preoperative GCS scores of 8 or less, 38 (65.5%) died while 12.1% and 13.8% had moderate disability and good recovery respectively (P=0.000). By contrast, only 4 (3.5%) deaths occurred in the 113 patients with scores ranging from 13 to 15 amongst who moderate disability and good recovery was achieved by 25.7% and 68.1% respectively.
Details of pupillary reaction to light were documented for 250 patients as follows: 199 patients (79.6%) had symmetrical reactive pupils, 28 (11.2%) had anisocore but reactive pupils and 23 (9.2%) had bilateral unreactive pupils. When pupillary characteristics were cross tabulated with outcome, 69.6% of patients with unreactive pupils died as compared to 7% of patients with bilaterally symmetrical reactive pupils (P=0.001). Further, 55.8% of those with bilateral reactive pupils achieved a functional recovery compared with only 14.3% and 8.7% of patients who had anisocore but reactive pupils and bilateral abnormal pupillary responses respectively (P=0.001).
Further, of the 155 patients with a history of loss of consciousness, 25.8% died during admission as compared to 8% mortality in patients with no history of loss of consciousness (P=0.018). In addition, functional recovery and moderate disability were achieved by 55% and 32% of those who remained conscious as compared to 40.6% and 23.9% of those who lost consciousness following trauma (P=0.018). A history of convulsions was associated with a lower rates of functional recovery 33.3% as compared to 17.348.4% of patients who did not have such a history (P=0.052).
Patients who underwent surgical drainage of hematomas had a higher rate of functional recovery (47.3%) and a lower mortality (17.6%) as compared to those who didn't who had functional recovery and mortality of 42% and 24% respectively (P=0.009). The length of time between the injury and operative decompression significantly influenced the final outcome. Patients who were operated on for less than 24 hours after the injury had a lower mortality (13.3%) than the patients operated on 2-4 days and >4 days after trauma who had mortality of 31.3% and 44.4% respectively (P=0.046). Furthermore, patients operated on <24 hours after trauma had a higher rate of functional recovery (66.7%) than other patients (P=0.046).
(See Table 1)
When the entire study population was subjected to logistic regression analysis, sex, age, pupillary reactivity, admission GCS scores and history of loss of consciousness were found to be significant independent predictors of functional recovery and mortality.
(See Table 2)
DISCUSSION
Acute subdural haematoma (ASDH) is still a condition with a high mortality and morbidity. The reported incidence of ASDH is as high as 5% in patients with head trauma and some retrospective studies report increased incidence with age (8). In spite of advances in neurotraumatology and aggressive neurosurgical intervention, the mortality rate of traumatic ASDH is still high in majority of series ranging between 39% (9) and 75% (10). Wilberger et al (11) reported that the overall mortality from traumatic ASDH is 66% and functional recovery 19%. In our series, the overall mortality was 20.1% and functional recovery 45.6%. This is similar to a hospital mortality rate of 21.75% that was reported by Tian et al (2) in their prospective Chinese series.
It has been established in literature that increasing age is associated with a higher mortality and lower likelihood of functional recovery from traumatic brain injury. Howard et al (12) compared 33 young patients (aged 18-40 years) with old patients (aged over 65) and they reported significantly higher mortality rate in the older group (74% versus 18%). Mosenthal et al (13) observed that the mortality from isolated traumatic brain injury for the geriatric population was twice that of younger patients. Munro et al (14) also found that patients aged 65 years and older had lower survival rates than patients less than 65 years old. Similar findings have been reported by other authors (7, 15, 16). In the study by Wilberger et al (11), the mean age of survivors was 41 years and of non survivors was 59 years. We observed a similar trend and found that age was an independent predictor of outcome in traumatic ASDH. In our study, a higher mortality rate (30.6%) was observed for patients older than 61 years (P=0.003), while patients aged 26 to 45 and less than 13 years had a mortality of 23.7% and 20 % respectively. Furthermore, fewer (24.5%) patients aged >61 years had good functional recovery as compared to 50% and 57.4% patients aged <13 and 14-25 years respectively (P=0.003). In addition, those patients older than 45 years showed significantly higher rate (OR=-0.12, P=0.036) of mortality by multivariate logistic regression analysis.
The mechanism by which age has such an effect on outcome is unknown, but suggestions include a poor regenerative capacity of the older brain and predisposition to develop a more lethal injury (12). Some of this increased mortality in the elderly may be explained by the intrinsic properties of the ageing brain, pre-existing co-morbidities and complications. Furthermore, the adverse effects of general anaesthesia and surgery may affect the respiratory and circulatory function of the elderly, increasing the severity of brain injury. Therefore, in addition to treating pre-existing diseases to decrease the risk of complications, improved long-term care should be emphasized for elderly surgical patients.
Pupillary abnormalities are associated with a significantly worse outcome. In our series, patients who had bilateral areactive pupils had a mortality of 69.6% as compared to 7% for patients with bilaterally symmetrical pupils (P=0.001). Further, 55.8% of those with bilateral reactive pupils achieved a functional recovery compared with only 8.7% of patients who had bilateral abnormal pupillary responses (P=0.001). Many authors reported that patients with bilateral fixed pupils at surgery had a mortality rate from 64 to 93% (6, 7, 11, 17, 18). Kim et al (19) reported that patients with one non-reacting pupil, had a mortality from 48 to 68%. This is in accordance with the findings of our series and is confirmed by other reports (20, 21, 22). In addition on logistic regression, pupillary abnormalities were strong predictors for mortality of patients with traumatic acute subdural hematomas (OR=-1.179, P= 0.000). It has been postulated that pupillary dilatation is associated with decreased brainstem blood flow and that ishaemia rather than mechanical compression of the third cranial nerve is an important causal factor (23). In addition, pupillary abnormalities also indicate brain herniation syndromes (2). Monitoring pupillary changes of trauma patients with coma is crucial to promptly detect any pupil inequality.
The time from the trauma until surgical decompression also affects the mortality. Some researchers have observed that the sooner surgery is performed in cases of acute head trauma, the better the final results are (24, 25). Seelig et al (26) in their study concluded that a delay from injury to operation was the factor of greatest therapeutic importance in traumatic ASDH. But the relationship between time to surgery and outcome is still controversial. Haselsberger et al (22) reported that 47% died and 32% had a favorable outcome among the patients operated within two hours after the onset of coma. On the other hand, Stone et al (21) reported no difference in patients operated within 4 hours of injury compared with those operated later. In our study, patients who were operated on for less than 24 hours after the injury had a lower mortality (13.3%) than the patients operated on 2-4 days and >4 days after trauma who had mortality of 31.3% and 44.4% respectively (p=0.046). Furthermore, patients operated on <24 hours after trauma had a higher rate of functional recovery (66.7%) than other patients (p=0.046). However in our series, due to the retrospective nature of the study, we were unable to analyse the length of the period of herniation or duration of operation as independent predictors. However, the mean time elapsing from accident to surgery was 3 days in our series which is much longer than that reported in various other studies. Taussky et al (1) documented a mean time elapsed of 3 hours in a Swiss population while Haselsberger et al (22) and Stone et al (21) reported mean times of 2 hours and 4 hours respectively.
CONCLUSION
This study has identified factors that influence outcome of patients with acute subdural hematomas in a Kenyan setup. An increased risk of death occurs in patients who are over 61 years of age and have lower preoperative GCS, the presence of pupillary abnormalities and a long interval between trauma and decompression. The findings would help clinicians determine management criteria and improve survival.
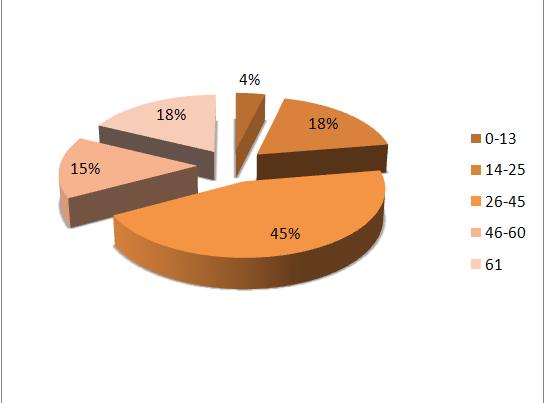
Figure 1


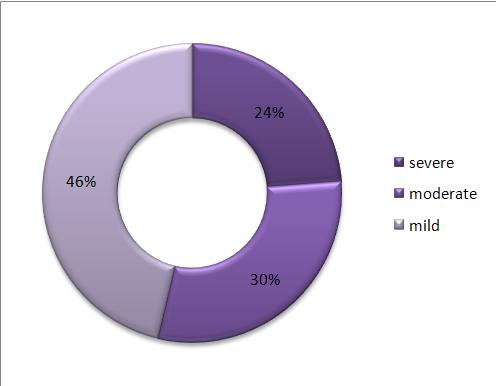
Figure 4