
|
|
|
CASE REPORT / CAS CLINIQUE
CEREBROSPINAL FLUID ASCITES. A CASE REPORT AND LITERATURE REVIEW
ASCITE ET LIQUIDE CÉPHALO-RACHIDIEN. A PROPOS D'UN CAS ET REVUE DE LA LITTÉRATURE
E-Mail Contact - MWANG’OMBE Nimrod Junius :
nimmag@hotmail.co.uk
ABSTRACT Cerebrospinal fluid ascites is one complication of ventriculoperitoneal shunt surgery. This case reports a 7year old child with abdominal distention five years after ventriculoperitoneal shunt insertion for hydrocephalus secondary to aqueductal stenosis. The child had a history of multiple shunt revisions. Liver, cardiac and renal causes of ascites were diagnostically ruled out. Cerebrospinal fluid biochemistry was normal but ascitic fluid biochemistry and electrophoresis of the ascitic fluid were deranged. The ascites resolved gradually within two weeks of endoscopic third ventriculostomy. Cases recorded in literature are reviewed in the discussion. KEY WORDS: cerebrospinal fluid (CSF) ascites, ETV, hydrocephalus INTRODUCTION Cerebrospinal fluid (CSF) ascites, the abnormal accumulation of CSF within the peritoneal cavity, must be distinguished from ascites due to hepatic, renal or cardiac disease. CASE REPORT A 7year old boy presented with one month’s history of progressive abdominal distention. Previously, the child had been managed for hydrocephalus secondary to aqueductal stenosis since 5month’s of age. VP shunting had been revised severally, with the latest one having been inserted 5years prior to the current illness. The patient took phenobarbitone for convulsions and had been seizure-free for over a year. Examination revealed a dyspnoeic child with a distended, tense, non-tender abdomen and a positive fluid thrill. Neurologic function was intact. Abdominal ultrasonography confirmed the presence of ascites and delineated normal appearance of the liver, kidneys and peritoneum, as well as good CSF flow through the VPS distal catheter. Serum kidney function values were within normal limits (urea 9.1, creatinine 61.7, Na+ 132, K+ 4.9) as were the liver function tests (total protein 68.7, albumin 37.4, AST 32.4, ALT 24.8, ALP 281.4, Dbil 4.8). Echocardiography revealed a normal functioning heart. The patient tested negative for HIVantibodies. CSF biochemistry was normal (glucose 5.6mmol/l, protein 0.2 g/l). On microscopy the CSF was clear and non-yielding of RBC, WBC, leucocytes or micro-organisms. Paracentesis identified a high protein transudate (Total protein 18.6 g/l, albumin 17.2 g/l) with normal glucose and reduced LDH values (6.9mmol/l and 62.4 U/l respectively). The serum ascites albumin gradient (SAAG) was 17 g/l. Pre-albumin was negligible on electrophoresis of the ascitic fluid; the other values were as follows: albumin 6 g/l, á1 globulin 5 g/l, á2 globulin 6 g/l, â globulin 9 g/l and ã globulin 1 g/l. Despite diuretic treatment and peritoneal tapping the ascites re-accumulated, but resolved two weeks following endoscopic third ventriculostomy (ETV). DISCUSSION Ascites following VPS surgery is seldom mentioned in literature. One retrospective study estimates it at 5.8% of all VPS complications (10). Only 28 cases had been recorded worldwide up until 1998(15). One day to 12 years is the documented interval between shunt placement and clinical ascites (5, 2, 9, 15, 8). Its little known aetiology(12) has led to proposed mechanisms; subclinical peritonitisthat hinders lymphatic drainage(2), elevation of CSF protein leading to peritoneal malabsorption (1, 14, 5) and CSF overproduction exceeding the absorptive capacity (9). Peritoneal irritation can be the result of multiple shunt revisions(3, 15), an immune reaction following vaccination(3) or shunt degradation(4, 8). High CSF protein is present in chronic infections eg tuberculosis(14) and brain tumours (eg optic gliomas and craniopharyngiomas)(5). Choroid plexus papillomas are a cause of ascites due to overproduction(9). Our patient had 6 VPS revisions between 2005 and 2007 secondary to VPS infection suggesting peritoneal irritation. The diagnosis of CSF ascites is made through various techniques; desialated â2 transferrin is a specific marker for CSF otorrhoea and rhinorrhoea with sensitivity and specificity of 100% and 95% respectively(8). Due to errors following the serial dilutions diagnostic technique(11) and possible alteration of ascites biochemistry depending on pre-diagnostic length of stay, feasibility of â2transferrin as a diagnostic test must be studied further. Electrophoretic patterns of normal CSF compared to serum should have a larger prealbumin peak, an absence of á2 macroglobulin and lower proportions of á1 and â globulins(6). Alpha 2 macroglobulin >4g/l and albumin < 32g/l are strong indicators of subclinical peritonitis (13). Raised LDH is a marker of malignancy in CSF(7). Our patient's ascites was a high-protein transudate with negligible pre-albumin, high á2 macroglobulin and low albumin levels. His LDH level was normal. The findings rule out malignancy and are in favor of subclinical peritonitis as the underlying cause of peritoneal malabsorption. Comparisons of the biochemistry of both CSF shunt aspirate and ascites from paracentesis strengthen a diagnosis of CSF malabsorption. CSF ascites resolves spontaneously following redirection of the CSF flow; by either ventriculoatrial conversion or ETV. In our case, ETV was performed, leading to resolution of symptoms within two weeks, in the absence of further peritoneal tapping or diuretic treatment. CONCLUSION In conclusion, the patient who presents with CSF ascites will have abdominal distention with no tenderness or neurologic deficit attributable to the ascites, could present months to years between the time the shunt is placed and the time symptoms evolve, will require multiple diagnostic tests to identify aetiology and can be helped by re-directing the flow of CSF. Our case falls short of the above by lacking the complete panel of diagnostic tests. It aims at sensitizing practitioners on this problem and on opening up further discussion of the topic. 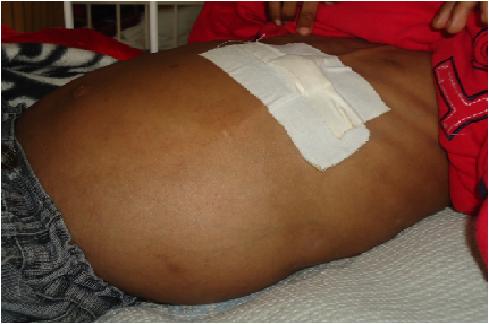 Figure 1 REFERENCES
|
© 2002-2018 African Journal of Neurological Sciences.
All rights reserved. Terms of use.
Tous droits réservés. Termes d'Utilisation.
ISSN: 1992-2647