|
|||
|
CLINICAL STUDIES / ETUDES CLINIQUES
MANAGEMENT AND FUNCTIONAL OUTCOME OF CHILDHOOD HYDROCEPHALUS AT THE KENYATTA NATIONAL HOSPITAL, NAIROBI
PRISE EN CHARGE ET EVOLUTION FONCTIONNELLE DE L'HYDROCEPHALIE DE L'ENFANT AU KENYATTA NATIONAL HOSPITAL, NAIROBI
E-Mail Contact - MUSAU Christopher Kyalo :
drckmusau@gmail.com
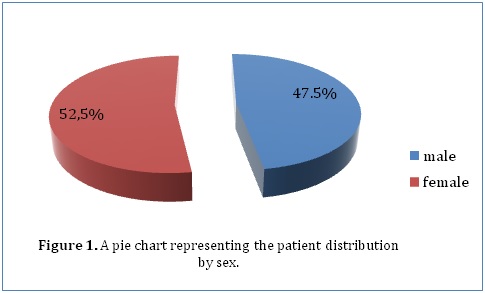
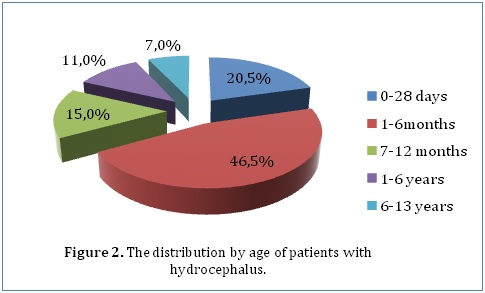
ABSTRACT Background Methods Results Conclusion Keywords: Functional outcome, Hydrocephalus, Pediatric neurosurgery Introduction Méthodes Résultats Conclusion Mots-clés: Hydrocéphalie, Neurochirurgie pédiatrique INTRODUCTION Hydrocephalus is a pathological condition caused by abnormalities of production or absorption of CSF in the brain. It is particularly common in infants and children. Hydrocephalus may be idiopathic or acquired. Most of the congenital cases are usually idiopathic in origin. Idiopathic hydrocephalus in children is commonly caused by meningitis, intracranial hemorrhage, brain tumors, trauma, and developmental anomalies of the brain. [1, 2] A number of studies have estimated the population incidence of congenital hydrocephalus to be between 2.5 and 8.2 per 10,000 live births. [3, 4, 5] The natural history of untreated congenital hydrocephalus is progressive cognitive decline and an early death, usually before the 3rd decade of life. [6, 7] The goal of this study was to evaluate the current management and outcome of children (aged 13 years and below) who were treated for hydrocephalus in a single institution in Kenya. The institution, Kenyatta National Hospital, is a tertiary teaching and referral hospital that has the largest neurosurgical unit in Kenya. METHODOLOGY After ethical approval from the Kenyatta National Hospital Ethics and Review Commission, the hospital records of 200 children (aged 13 years and below) who were admitted to the neurosurgical unit at the Kenyatta National Hospital and diagnosed with hydrocephalus between January 2000 and April 2013 were reviewed. In this retrospective analysis, the records were analyzed for demographic characteristics and clinical variables. Clinical variables included progressive enlargement of the head, irritability, negative changes in feeding habits, altered level of consciousness, headache, neck pain, visual disturbances, associated congenital anomalies, history of previous surgery for hydrocephalus, history of febrile illness or CNS infection, vital signs, AVPU score on admission, cranial nerve palsies, pupil abnormalities, bulging fontanelle, widening of cranial sutures and changes in motor tone. Management variables were also recorded, such as type of management whether surgical or medical with special emphasis on type of surgical intervention. Outcome was assessed according to the Glasgow Outcome Scale (GOS) score at the time of discharge from hospital. We intended to retrieve all the records in store of all patients (aged 13 years and below) who had ever been admitted to the neurosurgical unit at the Kenyatta National Hospital and diagnosed with hydrocephalus. The hospital only stored records going back to January 2000 and we managed to retrieve all of those which met our inclusion criteria (200 records). The data we obtained was collected in questionnaires, coded and analyzed using Statistical Package for Social Sciences (SPSS), version 18.0. Various variables were described using computed frequencies and means; discrete variables were compared using the x^2 test and continuous variables compared using Student’s t test. According to the GCS score determined upon admission, all patients were divided into three groups; those with GCS scores of 3-8, 9-13, and 13-15 for statistical analysis. Logistic and univariate linear regression models were run to determine which variables are independently associated with functional recovery and mortality. RESULTS Our study included 200 patients diagnosed with hydrocephalus at the Kenyatta National Hospital who met the inclusion criteria. Female patients accounted for 52.5% (105) of the study population while male patients accounted for 47.5% (95) (Figure 1). Our study only included patients who were age 13 years or younger. The mean age was 1.12 years (±2.513), with a range from 1 day to 13 years. The median age was 3.5 months. Majority of the patients (46.5%) were aged between 1 and 6 months, whereas 20.5% were younger than 28 days, 15% were aged between 7 months and 1 year, 11% were aged between 1 and 6 years and 7% were aged between 6 and 13 years (Figure 2). Only 28% (56) of the patients in our study had a health insurance cover.
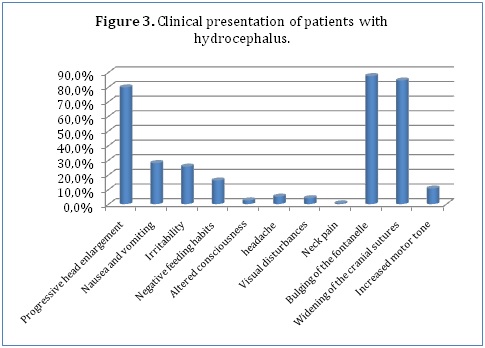
With regards to clinical presentation, majority of our patients (79.7%) presented with progressive head enlargement. A number of patients (28%) had a history of nausea and vomiting with 25.6% having experienced irritability by the time of presentation. 16.1% of the children had a negative change in feeding habits. Only 2.5% of our patients had altered consciousness (Figure 3). Due to the nature of the symptoms and ability to communicate them, only the older children were able to report headaches (5.1%), visual disturbances (4%) and neck pain (0.5%). Most of our younger patients had the characteristic bulging of the fontanel (87.4%) and widening of the cranial sutures (84.4%). Only 10.6% had increased motor tone.
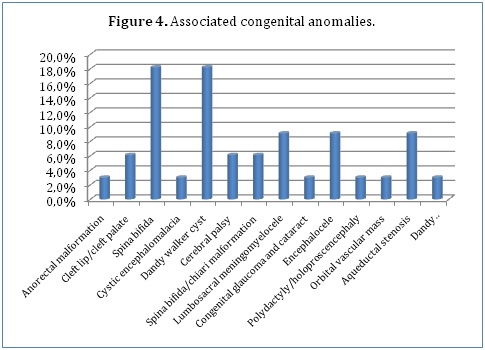
Some of our patients (16.5%) had associated congenital anomalies, the commonest being spina bifida (18.2%) and Dandy Walker cyst (18.2%). Other associated congenital anomalies included lumbosacral meningomyelocele (9.1%), encephalocele (9.1%), aqueductal stenosis (9.1%), spina bifida and chiari malformation combination (6.1%), cleft lip and cleft palate combination (6.1%) and cerebral palsy (6.1%). A few of our patients had anorectal malformations (3%), cystic encephalomalacia (3%), congenital glaucoma and cataract combination (3%), polydactyly and holoproscencephaly combination (3%), an orbital vascular mass (3%) and a Dandy Walker cyst, encephalocele and meningocele combination (3%) (Figure 4).
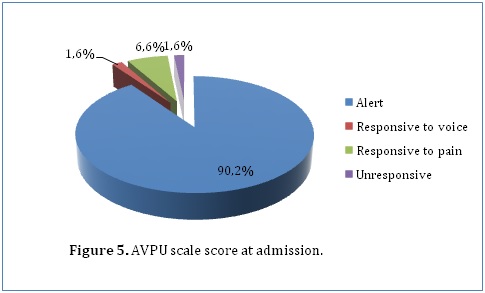
We also observed some risk factors in our study population which could lead to development of hydrocephalus. This included history of an unknown febrile illness (31.8%), history of central nervous system infections (23.7%) and a history of head trauma (1.5%). With regards to delivery, most of our patients were born through spontaneous vaginal delivery (67.7%) while 32.3% were born through caeserian section deliveries. Only 14% of our patients had delivery complications. This included perinatal asphyxia (42.9%), meconium aspiration (28.6%), prolonged or obstructed labor (14.3%), respiratory distress (7.1%) and breech delivery (7.1%). A minority of our patients (10.3%) had already undergone previous surgical treatment for hydrocephalus. The procedures they underwent included ventriculo-peritoneal shunting (94.4%) and endoscopic third ventriculostomy (5.6%). A few of the patients in our study who had undergone previous surgical procedures developed complications. This included mainly infections (37.5%), migration of the shunt (37.5%) and non-function of the shunts (25%). The AVPU scale on admission was recorded for 61 patients, among whom 55 (90.2%), 1 (1.6%), 4 (6.6%) and 1 (1.6%) patients were alert, responsive to voice, responsive to pain and unresponsive respectively (Figure 5). When outcome was cross-tabulated against admission AVPU scale score, it was observed that the proportion of patients with good functional recovery increased from 0% for patients who were unresponsive, to 100% for patients responsive to pain and voice and 92.1% for patients who were alert (P=0.017). Furthermore, percentage mortality significantly increased with decreasing AVPU scale score. Patients who were alert had a percentage mortality of 7.9% as compared to 100% in those who were unresponsive on admission.
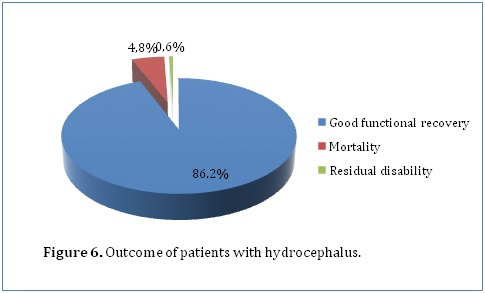
Pupillary reaction was recorded for 198 patients, with 5 (2.5%) having a positive history of abnormal pupillary responses. With regard to these patients, 94.4% of those with bilateral reactive pupils achieved a functional recovery compared with 100% of patients who had abnormal pupillary responses but this finding was not statistically significant (P=0.601). Details regarding loss of consciousness were available for 198 patients, with 5 (2.5%) having a positive history. Patients who did not have loss of consciousness were more likely to have good functional outcome (95%) as compared to those who did (80%) (P=0.117). In addition, patients who had loss of consciousness had higher percentage mortality (20%) than those who did not (4.4%). A number of investigations were done to diagnose hydrocephalus in our study population. This included ultrasound scans in the younger population with open fontanels. 25% of our study population had an ultrasound scan done which showed enlarged ventricles. Computed tomography scans were done in 44.5% of our study population. They showed enlarged ventricles (79.3%), acqueductal stenosis (27.1%), space occupying lesions (9.6%) and herniation (1.4%). 1 patient had an MRI done which showed enlarged ventricles. Glasgow Outcome Scale score (GOS) was recorded for 166 patients. Good functional recovery (GOS of 5) was achieved by 157 (94.6%) of these patients whereas residual disabilities (GOS of 2-4) accounted for 0.6% and mortality (GOS of 1) accounted for 4.8% (Figure 6). The likelihood of having a functional recovery did not vary with gender. Both males and females had an equal functional recovery rate of 94.6% although 1.4% of the male patients had residual disabilities as compared to 0% of female patients. However, females had a slightly higher mortality (5.4%) as compared to males (4.6%).The proportion of patients who achieved functional recovery seemed to increase with increasing age. Patients aged between 1 and 13 years (100%, P=0.070) were more likely to have good recovery as compared to patients younger than age 1 year. Those younger than age 28 days had a good functional recovery of 88.9% while those aged between 1 month and 6 months and those aged between 7 months and 1 year had a functional recovery of 94% and 96% respectively. In addition, mortality was also higher in those younger than age 28 days at 11.1% as compared to the other age sets. Those aged between 1 month and 6 months and those aged between 7 months and 1 year had a mortality of 4.8% and 4% respectively. There was no mortality in those older than 1 year.
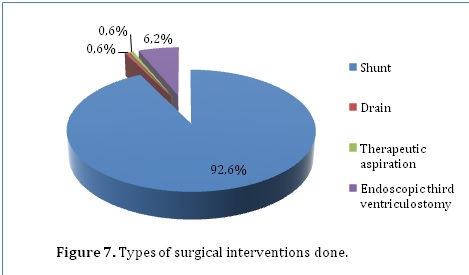
162 (81%) of the patients in our series had surgical intervention. Surgical interventions included shunts (92.6%), drains (0.6%), therapeutic aspiration (0.6%) and endoscopic third ventriculostomy (6.2%) (Figure 7). Patients who had surgical intervention were more likely to achieve functional outcome (95.7%) as compared to 50% in those managed conservatively. In addition, mortality was also higher among conservatively managed patients (50%) than surgically managed patients (3.7%) (P=0.000). However, residual disabilities were more common among the surgically managed patients (0.6%) than the conservatively managed patients (0%).
The mean duration of hospital stay was 19.72 (±42.543) days while the median duration was 8.5 days. Patients who stayed for a shorter duration had a better outcome (P=0.065). Those who stayed for 1 day or less and those who stayed for 2 to 10 days had a good functional recovery of 100% and 98% respectively. In contrast, those who stayed for 11 to 20 days and greater than 20 days had a functional recovery of 85% and 90.2% respectively. 2.6% of the patients in our series were admitted to our Intensive Care Unit (ICU). These patients had a poorer outcome with only 25% achieving good functional recovery, as compared to 96.2% functional recovery achieved by patients not admitted in ICU (P=0.000). Residual disabilities were also higher in ICU patients (25%) as compared to patients not admitted in ICU (0%). In addition, these ICU patients had a much higher mortality of 50% while patients not admitted in ICU had a mortality of 3.8%. DISCUSSION Outcomes following surgical management in pediatric patients with hydrocephalus have been evaluated in numerous studies around the world. To date, no authors have provided a study regarding outcomes in childhood hydrocephalus in Kenyan children in the African setup. The present study was performed to determine the outcome including surgical morbidity and mortality rates in children diagnosed with hydrocephalus in this setup. The male/female ratio in our study population (1.0:1.1) was lower than in other studied populations. [15, 16] Our population, however, was a non-selected group of all children (aged 13 years and below) who were diagnosed with hydrocephalus through the calendar years 2000-2013 at the Kenyatta National Hospital, Nairobi. This tertiary teaching and referral hospital has the largest neurosurgical unit in Kenya and therefore handles the majority of the hydrocephalus disease burden in the country through referrals. Our mortality rate of 4.8% was not comparable with the rate in other reports due to differences in follow-up. Our mortality rate was much lower, reason being that we evaluated our outcome at the point of discharge from the hospital. Other studies, such as one done by Paulsen et al [9], followed children with hydrocephalus for 20 years reporting a mortality rate of 22%. In our study, mortality was higher among conservatively managed patients (50%) than surgically managed patients (3.7%) (P=0.000). Only 5 of the 150 patients who had shunts inserted died thus recording a mortality rate of 3.3% in shunt-treated individuals. In a study done by Tuli et al [10], which studied shunt-treated children, the 10-year mortality rate was reported to be 12.4%. Lumenta and Skotarczak [11] reported a mortality rate of 13.7% in a long-term follow-up study (median 17 years, range 5-26 years) of congenital hydrocephalus. Our study reported that good functional recovery was achieved by all the 10 patients who underwent endoscopic third ventriculostomy (ETV). Better outcomes with ETV than with other treatment modalities were also reported by Vinchon et al [14], although they added that ETV likely benefited from selection bias and the fact that long-term figures are not available. A few of the patients in our study who had undergone previous surgical procedures developed complications. This included mainly infections (37.5%), migration of the shunt (37.5%) and non-function of the shunts (25%). In our series there were 5 deaths (2.5%) that occurred after shunt insertion. This may be attributed to shunt complications such as shunt malfunction and infections.[10] Shunt-related deaths in other hydrocephalic populations have demonstrated rates of 2%-5%, which may support our allegations. [12, 16, 17] It is therefore important for every neurosurgeon to closely monitor patients with shunts in order to ensure early detection of shunt malfunction and infections. Late recognition of these complications may result in death therefore providing a guideline regarding shunt failure symptoms to patients, family, and caregivers is also important and should be emphasized. According to our findings, factors that significantly influenced outcome included the level of consciousness at admission, type of management (surgical vs. conservative) and admission into our Intensive Care Unit (ICU). A lower AVPU score at admission was associated with a poorer outcome (P=0.017). Admission into our Intensive Care Unit was also associated with a poorer outcome (P=0.000). This may be because patients with severe neurological impairment were more likely to end up in our ICU. Conservatively managed patients had a poorer outcome as compared to surgically managed patients (P=0.000). A study done by Venkataramana et al [13] also had similar findings and went a step further to conclude that early CSF diversion and timely intervention results in better outcomes. They also reported that large ventricles (head circumference more than 50 cm), recurrent subdural collections and repeated shunt obstructions have a bad influence on the long-term outcome. [13] Recent studies done by Vinchon et al [14] and Bourgeois et al [18] added that epilepsy also has a serious negative impact on outcome. Our study has a number of limitations, including selection bias. We tried to mitigate this by retrieving all the records in storage instead of sampling. Because the primary registry was hospital-based and not population-based, our findings and conclusions may not apply to all individuals with childhood hydrocephalus within the general population. Whether our population represents a more or less severely affected group of individuals with childhood hydrocephalus is unknown. It is possible that patients with severe neurological impairment would be more likely to be included in our study because this hospital is a tertiary referral hospital. Patients with normal outcomes and no ongoing clinical problems may be less likely to be included in our study. In addition, this being a retrospective study, we relied on records but there were a few data variables which did not have complete data sets as illustrated by our results. Finally, since we only investigated outcome at the point of discharge from the hospital, more detailed follow-up studies are needed to identify the specific long-term outcomes of childhood hydrocephalus. CONCLUSION In our review of the management and outcome of childhood hydrocephalus in Kenyatta National Hospital, we have come to the conclusion that early CSF diversion when indicated improves outcome but vigilant monitoring for shunt complications is warranted. It was also noted that a lower AVPU score at admission and admission into our Intensive Care Unit were predictors of poor outcome. We recommend more detailed follow-up studies using population-based longitudinal data and quantitative quality-of-life measures to identify the specific long-term outcomes including the individual and social impacts of childhood hydrocephalus in Kenya.
REFERENCES
|
© 2002-2018 African Journal of Neurological Sciences.
All rights reserved. Terms of use.
Tous droits réservés. Termes d'Utilisation.
ISSN: 1992-2647