CLINICAL STUDIES / ETUDES CLINIQUES
HEREDITARY NEUROPATHY WITH LIABILITY TO PRESSURE PALSIES: A CLINICAL AND MOLECULAR STUDY IN A SOUTH AFRICAN FAMILY OF INDIAN DESCENT
NEUROPATHIE HEREDITAIRE AVEC HYPERSENSIBILITE A LA PRESSION : ETUDE CLINIQUE ET MOLECULAIRE DANS UNE FAMILLE SUDAFRICAINE D'ORIGINE INDIENNE
- Division of Neurology, Nelson R Mandela School of Medicine, University of Natal, Department of Neurology Wentworth Hospital Pvt Bag Jacobs 4026 South Africa
- Neurogenetics Laboratory, University of Lille, France
- Department of Neurology, University of Lille, France
ABSTRACT
Background
Hereditary neuropathy with liability to pressure palsy (HNPP) first described in 1947, has been showed to be due to a 1.5Mb deletion, which includes the peripheral myelin protein 22 (PMP22) gene, on chromosome 17p11.2. HNPP is more common than previously thought.
Objective
We describe the clinical and molecular features in a three generation family where the index case became acutely disabled following surgery for cervical spondylosis.
Method
A total of 14 (including the index case) were examined. Eleven had clinical evidence of disease. The disability of this group ranged from asymptomatic (1), mild (4), moderate (4), severe (1) to death (1). The findings on examination ranged from a single nerve involvement to a confluent mononeuropathy multiplex. The patient who died had marked proximal and distal weakness. The immediate cause of death is unknown. On history two individuals (now deceased) were said to be affected. A further 2 subjects who were not available for examination but who provided blood for molecular analysis were said to be normal.
A PCR based strategy for the determination of the PMP22 gene dose was undertaken in 15 subjects.
Results
Four of these individuals (2 on history and 2 on examination) who were clinically normal had no deletions. All the clinically affected individuals all exhibited the appropriate deletion. One was clinically normal but carried the deletion. Sample from a 16th individual, who was not examined, was insufficient.
Conclusion
This study is the first report of the existence of HNPP in South Africa. Cases are probably being missed. The correct diagnosis is important, as with appropriate measures and patient education, disability can be significantly reduced.
Keywords : Africa, Hereditary, Neuropathy,Pressure palsy, South Africa
RESUME
Introduction
Décrite en 1947, la neuropathie héréditaire avec hypersensibilité à la pression (NHHP) est une neuropathie héréditaire sensitivomotrice à transmission autosomique dominante. Cette affection est liée à un défaut de synthèse d’une protéine de la myéline : la protéine PMP22 (peripheral myelin protein 22) en rapport dans près de 90% des cas à une délétion de 1,5 mégabases dans la région 17p11.2 incluant le gène PMP22. L’HNPP paraît plus fréquent qu’on ne le pensait.
Objectif
Nous décrivons les aspects cliniques et moléculaires d’une famille sud africaine d’origine indienne, sur trois générations, découverte lors d’une décompensation aiguë suite à une intervention pour une myélopathie cervicarthrosique.
Matériel et méthodes
14 cas ont été étudiés. 11 patients avaient des signes cliniques évidents. Le groupe a été classé selon les aspects suivants : asymptomatique, discret, modéré, sévère et décès. Les constatations étaient en rapport avec une atteinte tronculaire nerveuse, unique ou multiple. . Une enquête familiale a permis de réaliser des examens cliniques et biologiques .
Résultats
Le PCR a été pratiqué chez 15 patients. Quatre des patients asymptomatiques n’avaient pas de délétion. Tous les patients symptomatiques avaient une délétion. Un patient asymptomatique était porteur d’une délétion.. Le patient décédé de cause inconnue présentait une faiblesse proximale et distale très marquée
Conclusion
Il s’agit du premier cas rapporté en République Sud-Africaine sans que cela ne préjuge du nombre de cas qui est vraisemblablement sous-estimé.
Le diagnostic de HPPN ne doit pas être omis compte tenu des éventuelles conséquences fonctionnelles.
INTRODUCTION
Hereditary neuropathy with liability to possible palsies (HNPP) was first described by De Jong in 1947 (2) In 1964 Earl et al (3) showed that it was transmitted through an autosomal dominant gene. In HNPP, recurrent peripheral nerve palsies occur because of minor compression trauma. Commonly affected nerves are the radial, median and peroneal nerves. Electrodiagnostic tests demonstrate significantly reduced velocities at compression sites of the peripheral nerves. Teased fibre preparation show sausage like formations called tomaculae of the peripheral myelin.
In 1993 Chance et al (1) showed that HNPP was due to 1.5Mb deletion in the 17p 11-2 region spanning the gene for peripheral myelin protein 22 (PMP-22), which was confirmed by others (4,7,10)
We describe a South African family of Indian descent where the index case inadvertently underwent surgery for presumed cervical spondylosis and developed extensive weakness post operatively.
PATIENTS AND METHODS
The index case (III4) was a 36 year old man who gave a 10 year history of pain in the neck and shoulders with radiation down the right arm to the thumb and index finger. About 2 years prior to referral he developed numbness of the middle three digits of the right hand. Six months prior to referral there was acute weakness of the right hand. A CT myelogram done elsewhere showed mild indentation of the thecal sac at C6/C7 disc level, which was operated on. When he awoke from the surgery he noticed total numbness and paralysis of both legs and weakness of the left arm. He gradually improved over the ensuing months but one month prior to referral he again developed acute weakness of the left arm and a right wrist drop. There were no thickened nerves. Weakness was noted in the distribution of both radial nerves, right median nerve and the deep branches of both common peroneal nerves. All the tendon jerks were present and equal. Touch was impaired in a glove and stocking distribution. Pinprick was impaired in a patchy distribution in the limbs. Joint position and vibration sense were intact.
Review of the family history confirmed an autosomal dominant pattern of neuropathy. A total of 14 members over three generations were examined. In 11, the history and clinical features were consistent with a liability to pressure palsies (Figure).
Blood was collected from 16 members after informed consent but the sample was insufficient in one individual (II14). The presence of a deletion on chromosome 17p 11.2 containing the PMP22 gene was determined by gene dosage analysis as previously described7.
A sural nerve biopsy done previously on one of the affected individuals (II8) was available for review.
RESULTS
Genetic analysis
The PMP22 gene was deleted in all of the clinically affected individuals. Another subject was clinically normal (III3) but showed a deletion. Four normal individuals, 2 on history (III1 and III2) and two on examination (III8 and III13) did not show any deletions (Figure 1).
Nerve biopsy
The semi-thin Toluidine blue sections and the electron microscopy of the sural nerve biopsy showed abnormally thick myelin, double myelin sheaths as well as thinly myelinated large axons (Figure 2a and 2b).
DISCUSSION
HNPP presents as an isolated neuropathy or as a mononeuropathy multiplex following relatively minor trauma or compression of the nerve for a short duration. The index case gave a history suggestive of a mononeuropathy multiplex and then had serious disability following unwarranted surgery. Other family members had varying degrees of disease. The features noted in this family mirror those described in the early literature.
Electrophysiological tests demonstrate reduced conduction velocities, prolonged distal latencies and conduction blocks at typical sites of compression. The one patient (II8) in our family who had the nerve biopsy also had electrophysiological tests with typical findings. He was initially suspected to have chronic inflammatory demyelinating Polyneuropathy. The sural nerve biopsy in this patient showed the features similar to that reported.
HNPP and Charcot-Marie-Tooth 1A (CMT1A) illustrate well the effect of gene dose.
Duplication of the 17p11.2 locus results in the clinical features of CMT1A whilst deletion results in HNPP. Animal models have been genetically engineered to over-express PMP22, giving rise to a CMT1A phenotype (11) Similarly, a knockout mouse model (8)demonstrating the HNPP phenotype has been developed.
Recent human HNPP studies have shown both phenotypic and genotypic heterogeneity. Mancardi et al (6) described 3 patients with progressive sensory-motor Polyneuropathy with tomaculous changes on biopsy. In all the patients the 17p11.2 deletion was demonstrated. The typical HNPP presentation may also occur with small deletions and distinct mutations rather than the 1.5Mb deletion of the PMP22 gene. At least seven point mutations in the PMP22 gene have been reported, reviewed by Stögbauer et al (12)
PMP22 is a transmembrane protein which is widely expressed, but the highest levels are found in the peripheral nerves where it constitutes approximately 5% of total protein content (14)
It carries the HNK-1 epitope and therefore has the characteristics of an adhesion molecule. Whilst the exact function of PMP22 remains unclear, experimental evidence suggests the protein promotes myelin formation at early stages of myelinogenesis and influences thickness and stability of myelin at the later stages (14)
HNPP has been described in many parts of the world including in USA, UK, Finland, India, Australia and Japan (13). Our study is the first report of HNPP in a South African family of Indian descent. The clinical and genetic findings are similar to those described in other parts of the world.
CONCLUSION
HNPP is probably more common than previously thought. Careful history taking is vital. HNPP should be considered in any unexplained neuropathy even in the absence of a family history because of high mutation rate in sporadic cases (5). HNPP should be excluded in all patients with recurrent demyelinating mononeuropathies.
| ACKNOWLEDGEMENTS |
| Thanks are due to the family members for participating in the study and to Ms. Enstrom and Ms. Seeramaloo for secretarial assistance. |
Table:
| SUBJECT NUMBER |
GENDER |
CLINICAL FINDINGS |
PMP 22 DELETION |
| 311 |
M |
Multiple neuropathies |
Y |
| 312 |
F |
Foot drop |
Y |
| 313 |
M |
Recurrent foot drop |
Y |
| 314 |
M |
Bilateral foot drop |
Y |
| 315 |
M |
Recurrent foot drop |
Y |
| 316 |
F |
Asymptomatic foot weakness |
Y |
| 318 |
M |
Normal |
Y |
| 319 |
F |
Normal |
N |
| 320 |
F |
Normal |
N |
| 321 |
M |
Not examined |
Insufficient |
| 322 |
M |
Not examined |
Y |
| 323 |
F |
Not examined |
Y |
| 324 |
F |
Quadriparesis |
Y |
| 325 |
M |
Polyneuropathy |
Y |
| 326 |
M |
Not examined |
N |
| 327 |
F |
Not examined |
N |
This table lists the gender and clinical presentation of individuals who had genetic testing.
Y = Yes
N = No
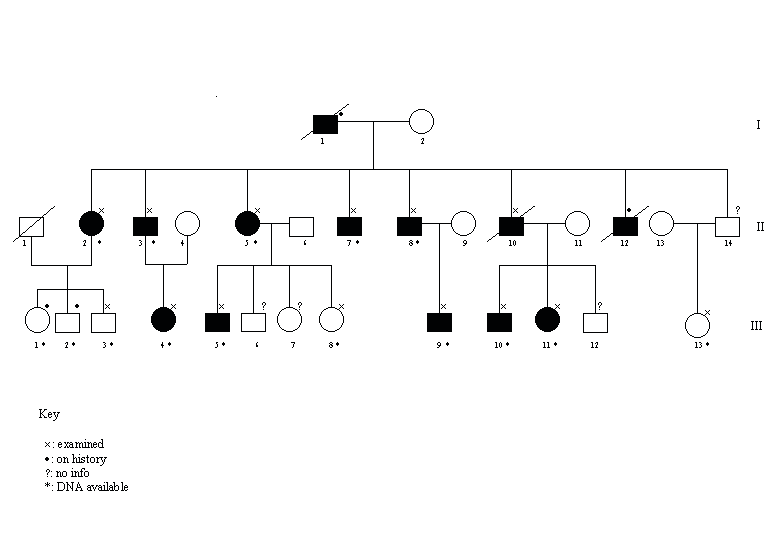
Figure 1:
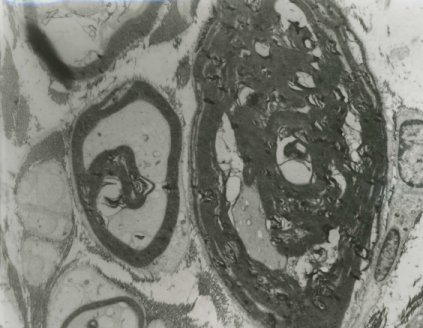
Figure 2a
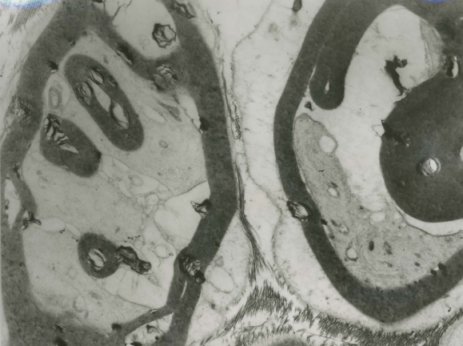
Figure 2b
REFERENCES
- Chance PF, Alderson MK, Leppig KA et al. DNA deletion associated with hereditary neuropathy with liability to pressure palsies. Cell 1993; 72: 143-151.
- De Jong JGY. Over families met hereditaire dispositie tot het optreden van neuritiden, gecorreleerd met migraine. Psychiatr Neurol 1947; 50:60-76.
- Earl CJ, Fullerton PM, Wakefield GS, Schutta HS. Hereditary neuropathy with liability to pressure palsies. A J Med 1964; 132: 481 – 498.
- Le Guern E, Sturtz F, Gugenheim M et al. Detection of deletion within 17p11.7 in 7 French families with hereditary neuropathy with liability to pressure palsies (HNPP). Cytogenet Cell Genet 1994; 65: 261 – 264.
- Lupski JR. Charcot-Marie-Tooth Polyneuropathy: Duplication, gene dosage and genetic heterogeneity. Pediatric research 1999; 54(2): 159 – 165.
- Mancardi GL, Mandich P, Nassani I et al. Progressive sensory-motor polyneuropathy with tomaculous changes is associated to 17p11.2 deletion. J Neurol Sci 1995; 131: 30-34.
- Mariman ECM, Gabreëls-Festen AAWN, van Beersum SEC et al. Gene for hereditary neuropathy with liability to pressure palsies (HNPP) maps to chromosone 17 at or close to the locus for HMSN type 1. Hum Genet 1993; 92: 87-90.
- Maycox PR, Ortuno D, Burrola P et al. A transgenic mouse model for human hereditary neuropathy with liability to pressure palsies. Molec Cell Neurosci 1997; 6: 405 – 416.
- Poropat RA, Nicholson GA. Determination of gene dosage at the PMP22 and androgen receptor loci by quantitative PCR. Clin. Chem 1998; 44(4):724-730.
- Reisecker F, Leblhuber F. Lexner R, Redner G, Rosenkranz W, Wagner K. A sporadic form of hereditary neuropathy with liability to pressure palsies: clinical , electrodiagnostic and molecular genetic findings. Neurology 1994; 44: 753 – 755.
- Stögbauer F, young P, Kuhlenbäumer G, De Jonghe P and Trimmerman V. Hereditary recurrent focal neuropathies. Neurology 2000; 54: 546 – 551.
- Sereda M, Griffiths I, Pühlhoger A et al. A transgenic rat model of Chacot-Marie-Tooth disease Neuron 1996; 16: 1049 -1060.
- Van Wensen PJM. Hereditary neuropathy with liability to pressure palsies in Ed de Jong, Hereditary neuropathies and spinocerebellar atrophies. Handbook of Clinical Neurology, Elsvier, Amsterdam 1991; 16 (60): 61-70.
- Welcher AA, Suter V, De Leon M, Snipes GJ, Shooter EM. A myelin protein is encoded by the homologue of a growth arrest-specific gene. Proc Natl Acad Sci USA 1991; 88: 7195 – 7199.



