
|
|
|
CLINICAL STUDIES / ETUDES CLINIQUES
PITUITARY ABSCESS
E-Mail Contact - SEMPLE Patrick Lyle :
psemple@uctgsh1.uct.ac.za
SUMMARY Pituitary abscess is a rare disease that remains very difficult to diagnose preoperatively even with newer imaging techniques such as CT – and MRI scanning. We describe four cases, of which only one was diagnosed pre-operatively. Pituitary abscess may present as meningitis in a patient with enlarged sella on skull x-rays. More commonly it is indistinguishable from a pituitary tumour both clinically and radiologically and the diagnosis comes as a surprise at surgery. Treatment is by transsphenoidal drainage, appropriate antibiotic and replacement therapy as indicated. RESUME Les abces hypophysaires sont rares et demeurent tres difficile a diagnostiquer en pre-operatoire, malgre les nouvelles techniques d’imagerie medicale comme ie scanner et I’IRM. Nous decrivons 4 cas parmi lesquels 1 seul a ete diagnostique en pre-operatoire. Les abces hypophysaires peuvent avoir les memes signes cliniques qu’une meningite chez les patients ayant une selle turcique elargie a la radiographie cranienne. Il est generalement tres difficile de differencier a , l’examen clinique et radiologique, les abces des tumeurs hypophysaires. Le diagnostic est souvent pose au cours de l’intervention chirurgicale. Le traitement consiste en un drainage transsphenoidal, une antibiotherapie appropriee, et une therapeutique de substitution selon le besoin. Keywords : Pituitary abscess, pituitary infection, transsphenoidal drainage, Africa INTRODUCTION Pituitary abscess is an uncommon condition, even in an environment where intra-and extracranial sepsis is common. Since Simmonds published the first case of a pituitary abscess in 1914 [17] over 60 cases have been described. MATERIALS AND METHODS The Department of Neurosurgery at Groote Schuur Hospital managed 4 patients with this diagnosis between 1979 and 1995 and during the same period operated on 496 pituitary adenomas. Hospital records and neuroradiological reports were the sources of information about the cases presented here. Case 1 This 21 year old male presented in 1979 with 1 month’s history of headache which had become generalized and was unassociated with photophobia. As a child he was injured with a sharp object over the bridge of his nose. Six months prior to presentation he hit his head against the windscreen in a motor vehicle accident and may have had rhinorrhoea for about a week. He suffered intermittent headache from that time. On examination he was confused, drowsy and had neck stiffness but was apyrexial and his ears were normal. No abnormality was found on systemic examination and the cranial nerves and peripheral nervous system were intact. Haematological examination revealed a haemoglobin of lOg/dl, white cell count of 8000 and ESR of 60. A lumbar puncture yielded CSF at a pressure of 50 cm of water and was clear and colourless. Laboratory analysis of the CSF revealed: protein 5g/dl, globulin l+, glucose 2,5, polymorphs 87 and lymphocytes 97. Gram’s stain was, however, negative and there were no yeast’s on Indian ink staining. A remarkably enlarged sella with a thin dorsum sellae and opacification of the posterior part of the sphenoid sinus was shown on skull-xray. Tomography confirmed the sellar enlargement but CT scan showed no abnormality apart from this. Lumbar air encephalogram demonstrated a soft: tissue mass involving the sphenoid sinus, pituitary fossa and suprasellar region. On carotid angiography displacement of the A I segment upwards and the siphon laterally, but no tumour blush, could be seen. Because the patient presented with a sterile meningitis in association with an enlarged pituitary fossa and evidence of a soft tissue mass involving the sphenoid sinus and pituitary fossa region, the diagnosis of an infective lesion was made but no aetiological factor could be implicated.
At transsphenoidal exploration the sphenoid sinus was found to be occupied by yellowish chronic granulation tissue so that a craniopharyngioma was also suggested. Sinus aspiration yielded nothing but when the needle was advanced into the pituitary fossa, 4 ml of thick pus was obtained. The entire cavity was cleared of its purulent content, washed out with an antibiotic solution and using the image intensifier it was confirmed that the dome of the abscess had completely come down to the floor of the fossa. The post-operative course was unremarkable u2013 the only complication was a low serum sodium due to inappropriate ADH secretion. Case 2 This patient, a 76 year old man, had a background history of being treated for hypothyroidism and hypopituitarism for 11 years, and had been fully investigated for this 5 years prior to presentation. A lumbar air encephalogram showed no suprasellar extension of a pituitary mass at that stage and the lumbar CSF was normal. He presented to our department in 1982 with a history of severe headache and neck stiffness with episodes of reduction in his level of consciousness and a bilateral decrease in visual acuity for 4 months. He also had diplopia in all directions of gaze for 3 months. On examination no systemic abnormality was found. He was alert and fully conscious with no neck stiffness. The right pupil was slightly larger than the left but both were fully reactive. A decrease in visual acuity was noted and visual field tests revealed bitemporal hemianopia, fundi were normal. Full blood count, biochemistry, ESR and liver function tests were all normal. Tomography of the pituitary fossa suggested a non-invasive asymmetrical lesion within the fossa. CT scan identified a small isodense non-enhancing mass bulging upwards into the chiasmastic cistern but with no extension into the sphenoid sinus. At transsphenoidal exploration, the mucous membrane of the sphenoid sinus was found to be thickened, the sellar floor very thin, and on opening this, a copious volume of pus was drained. Culture of the pus showed no organisms and no acid fast bacilli were grown. He made an uneventful recovery and was soon back on his maintenance steroids. Case 3 A 24 year old female school teacher presented in 1990 with a years’s history of amenorrhoea, galactorrhoea, frontal headaches and increasing visual problems. Six months prior to referral to our department she was found to have an upper quadrantic bitemporal hemianopia and the prolactin levels were found to be mildly elevated. CT scan revealed a pituitary mass with suprasellar extension. 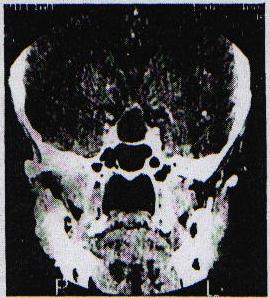 Figure 2 A prolactinoma was diagnosed and she was commenced on bromocriptine. There was, however, no improvement over the next six months, despite the fact that her prolactin was now in the normal range. There was no past history of note. Apart from bitemporal hemianopia, which was more extensive on the left, her clinical examination was non-contributory except for an ESR of 82 mm. At transsphenoidal exploration incision of the dura revealed pus. The cavity was adequately drained and the patient had an unremarkable post-operative course. The Gram’s stain revealed gram positive cocci but there was no growth on culture. On a follow-up CT scan in 1993 the abscess had, however, reformed. Clinically the patient was completely well and neurological examination, including the visual fields, was normal. As the patient was reluctant to undergo surgery she was placed on a 3 month course of antibiotic therapy. Follow up CT scan in 1994 revealed the abscess to be slightly larger, and it was re-evacuated transsphenoidally with a small drain left in situ for a few days to ensure complete drainage. She has subsequently made a good recovery and the abscess has not recurred on MRI scanning. Case 4 In 1992, this 41 year old male developed headaches and blurring of vision associated with severe thirst, polydipsia and polyuria. He also experienced sleep problems with snoring and excessive night sweats. His weight had increased and he had become impotent. On examination he was overweight, apyrexial with normal secondary sexual characteristics but hypogonadism was noted. Systemic examination and examination of the nervous system were normal with no papilloedema or visual field defect. Investigation revealed that his glucose tolerance test curve was impaired and the ESR was 32 mm. Endocrine testing revealed normal prolactin, thyroid hormone and FSH but testosterone was low. Testing of visual fields showed no evidence of chiasmal compression. On CT scanning a low density pituitary mass with slight suprasellar extension was demonstrated. MRI brain scan confirmed a mass lesion in the pituitary fossa with upward displacement of the optic chiasm. There was an area of enhancement with Gadolinium in the right upper aspect of the pituitary fossa. These features were brought to be in keeping with a pituitary macro-adenoma. 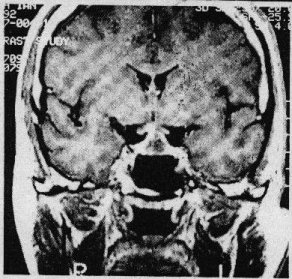 Figure 3 The patient was stabilized on Desmopressin preoperatively. On opening the dura at transsphenoidal exploration, pus was encountered. No pituitarys adenoma was found and after appropriate specimens for bacteriological assessment were taken, the abscess cavity was irrigated and closed. Staphylococcus Aureus was grown on culture and the appropriate antibiotic was commenced. The post operative course was uneventful. DISCUSSION The diagnosis of pituitary abscess remains a vexing clinical problem. It is rarely seen in acute phase by experienced practitioners so that a pre-operative diagnosis is hardly ever made and a surprise finding at surgery or autopsy is often the course of events. CLINICAL PRESENTATION No age or sex prevalence is evident in the world literature on this condition [1,3,10]. Symptoms usually develop over a few months but in a few cases very acute evolution has occurred [10]. The mass effect may cause headache and visual loss gradual loss of vision due to extension of the tumour above the level of the diaphragma sellae is usual, but may be as acute as in pituitary apoplexy [16]. Endocrine deficiency may or may not be present, depending on the duration of the disease and the degree of compression of the pituitary. The presentation of the pituitary abscess may, therefore, be distinguishable from that of a pituitary tumor 114]. Indeed, the abscess may develop within a primary pituitary tumor or cyst [16]. It has been stated, however, that the development of diabetes insipidus may help differentiate a pituitary abscess from a tumour. Diabetes insipidus only develops in 10% of pituitary adenomas but commonly occurs in the evolution of pituitary abscesses [9] and metastatic tumours of the pituitary. The presence ol combined anterior and posterior pituitary disturbance in the absence a hypothalamic lesion should also arouse the suspicion of an abscess [1]. Three of our patients presented with a combination of headache, visual disturbance and endocrine dsyfunction thought to be compatible with a pituitary adenoma. Significantly, the one patient had Diabetes insipidus as his presenting complaint. There may be associated meningitis and this feature in the presence of a large sella due to a known or suspected pituitary tumour should raise the possibility of this diagnosis [1,3,6,8). It has, however, been noted that less than half of the patients with a pituitary abscess will have a meningitic picture [3]. Sellar enlargement associated with pituitary failure and intermittent attacks of meningitis may, however, also be a feature of Rathk cleft cysts. On the other hand, there may be a remarkable absence of any meningitic reaction in the presence of a florid sellar abscess [14]. Our one patient presented with a meningitis following a skull base fracture and also had a large sclla on skull x-ray. The most common way a pituitary abscess presents is as a surprise finding at surgery [3]. AETIOLOGY A sellar abscess may follow previous surgical exploration [3] or Yttrium implantation and in the latter instance, aspergillosis is a particular common causal organism [7]. It may occur in a preexisting chromophobe pituitary adenoma 14,11,18,19] or a Rathke’s cleft cyst [12]. Dominique and Wilson [6] stated that pituitary tumors become susceptible to infection because of impaired circulation, areas of necrosis, or local immunological impairement. It may result from local extension of chronic sphenoid sinusitis, [3,19] but this is probably very rare.Our one patient (Case 1) developed a pituitary abscess following a probable basal skull fracture suggesting this as a possible post-traumatic complication. This patient as well as patient no. 2 had evidence of sphenoid sinus infection. In the past a pituitary abscess as a feature of a septicaemic state was noted [8,17], but this has become extremely rare. Most commonly it seems to arise spontaneously. MICROBIOLOGY Gram’s stain was done on all specimens which showed the typical features of pus. Staphylococcus Aureus was cultured from case 4 and the other three cultures were negative although gram positive cocci were seen in case 3. The organisms are almost always bacterial, although the occasional fungal abscess has been reported [14] and this has been particularly so in patients who had. Yttrium implantations, probably due to the immuno-compromised state of the patient [6]. In more than half the reported cases no organism was cultured, but anaerobic organisms were not always looked for [1,8,11,16]. In addition the finding of sterile pus may also be due to the use of antibiotics prior to surgery. Organisms identified are unremarkable but may be of low virulence or usually non pathogenic [10,16]. DIAGNOSIS Diagnosis is difficult pre-operatively as there may be very little to suggest an infective process. One of our patients had a normal sedimentation rate and only one was febrile. CSF analysis may be abnormal although not invariably so [14] and is not frequently done. Plain radiology of the skull always shows an enlarged sella turcica [2,3,8,9]. The fact that the sella is enlarged indicates some chronicity of this lesion and if associated with pituitary failure, implies that the process has been present for a considerable time. A variety of CT scan have been described with pituitary abscess. A low density intrasellar mass with a cystic appearance and marginal contrast enhancement are almost always demonstrated, but these features are not specific to pituitary abscess. We have encountered them in a infarctive pituitary apoplexy [5]. Features that may be more specific are permeative destruction of the sella floor, heterogenecity of the cyst contents suggesting the presence of debris in the cyst and cystic dilatation of the infundibulum [1]. CT scans in apost-surgical patient are particularly difficult to interpret in the diagnosis of an abscess [6]. MRI scan performed in one of our patients showed an area of enhancement compatible with a microadenoma. MRI has been found to be superior to CT scan in 41 % and equal in 54% of cases of sellar and parasellar masses [9], but has not as yet been proved to be a better diagnostic tool for the diagnosis of a pituitary abscess. TREATMENT In a patient presenting with meningitis and an enlarged fossa which suggest the diagnosis, prompt treatment with antibiotics should follow. If this does not result in immediate improvement, the sella should be explored and the abscess drained transsphenoidally. This route allows drainage without risk of contaminating the subarachnoid space [6,11,19]. PROGNOSIS Robinson has stated that the major cause of poor prognosis is delay in diagnosis and surgical treatment [15]. Pituitary abscess has an overall mortality of 28%, however those patients with meningitis have poorer outcome with a mortality of 45% [6]. CONCLUSION Pituitary abscess is a rare but potentially fatal condition. It may present as a meningitic illness or as a sellar mass lesion with endocrine and visual abnormalities. There is an enlarged sella on skull x-ray and cystic mass lesion on CT scan or MRI. Prompt treatment with antibiotics, steroids and transsphenoidal drainage should ensure good recovery for the patient. The majority of pituitary abscesses will, however, probably still be a surprise finding at surgery. REFERENCES
|
© 2002-2018 African Journal of Neurological Sciences.
All rights reserved. Terms of use.
Tous droits réservés. Termes d'Utilisation.
ISSN: 1992-2647
