
|
|
|
CASE REPORT / CAS CLINIQUE
ANDERSEN SYNDROME : DESCRIPTION OF A CASE
ANDERSEN SYNDROME : DESCRIPTION D'UN CAS
E-Mail Contact - GURER Reyhan :
reyhan_gurer@yahoo.com
SUMMARY A 45 year-old man with periodic muscular weakness is presented. He had 4 similar transient generalized weakness epizodes since the age of 38. His initial evaluation showed mild proximal weakness with no clinical or electrical myotonia. The serum potassium level was 2.7 mEq/lt. He showed dysmorphic features with short stature, a hypoplastic mandible, hypertelorism, low set ears, micrognathia, bilaterally short index fingers, broad forehead. His three children had almost the same dysmorphic features Key words : Potassium, dysmorphic features, weakness, Andersen syndrome INTRODUCTION In 1971, Andersen et al [1] described an 8-year-old boy with a distinct syndrome marked by clinical triad of PP, cardiac dysrhythmia and developmental anomalies named as Andersen syndrome (AS). The attacks are often superimposed on a background of generalized weakness. The inheritance pattern appears to be autosomal dominant with significant phenotypic heterogeneity. The mutations affect a potassium channel, called Kir 2.1-a member of a large family of potassium channels that help regulate the flow of potassium out of muscle cells [5]. REPORT OF CASE A 45-year-old man with periodic, sudden onset, generalized weakness is presented. From his detailed history, it was learned that he had 4 similar transient generalized weakness epizodes since the age of 38. The epizodes were of variable duration from 1 to 3 days and were either spontaneous or induced by prolonged inactivity or following meals and rest after exercise. There was no family history of systemic or neurological illness except his fathers’ death of hearth failure at age 38. Physical examination of the patient revealed prominent dysmorphic features such as hypoplastic mandible, hypertelorism, low-set ears, micrognathia, broad forehead [figure1], bilateral short index fingers [figure 2] and short stature. Also similar dysmorphic features were recognized from his father’s photograph [figure 3]. He had 3 children and their physical examination also revealed almost the same but milder dysmorphic changes [figure 4,5,6]. The patient’s blood pressure and pulse rate were normal. On neurological examination cranial nerves were intact. Generalized proximal muscle weakness with more prominent in lower extremities was found. Tendon reflexes were decreased, sensation was normal. There was no clinical myotonia. Full blood counts and biochemical examinations were performed and no abnormality was seen except the potassium level. His initial potassium level was 2.7 mEq/lt. After infusion of 1000 cc Isolyte M solution (includes 0.15 g potassium) to the patient in 24 hours’ time, potassium level increased to 4.5 mEq/lt. And then his neurological examinations became to normal state. Cardiac examinations including electrocardiography (ECG), echocardiography and Holter monitoring were unremarkable. Brain and spinal cord magnetic resonance imaging were normal. Electromyography (EMG) of limb muscles showed no evidence of myotonia. Muscle biopsy from deltoid muscle, showed mild myopathic findings with chronic denervation. Hypokalemic challenge with intravenous glucose (2 gm/kg) followed 30 minutes later by insulin (0.1 unit/kg intravenously) resulted in no change in muscle strength despite a decrease in serum potassium from 4.3 to 3.7 mEq/lt. Potassium challenge was not performed, because of danger of fatal cardiac complications. DISCUSSION Andersen’s syndrome is characterized by periodic muscle paralysis, cardiac arrhythmia and abnormal growth that includes short stature, and deformations of the spine, fingers, toes and face [6]. Weakness occurs episodic, lasting one hour to days, attacks may be rare though some patients may have persistant generalized background weakness, onset from early childhood to anytime in adulthood. The beginning of the attacks in our patient was at the age of 38. It was slightly a long time for the occurence of the symptoms. Hypokalemia, potassium administration, exercise are the precipitants for attacks or it may be idiopathic. In AS, potassium shifts during attacks of weakness are inconsistent. Recognition of the characteristic face in AS permits an early diagnosis and the detection of the severe systemic manifestations associated with the syndrome. Severity of the facial involvement does not correlate with the the severity of heart or muscle involvement [2]. The patient’s electrophysiological examination included nerve conduction study and electromyography (EMG) exclude the presence of myotonia and muscle biopsy showed mild myopathic findings with chronic denervation. In periodic paralysis muscle biopsy may show a wide range of abnormalities, vacuoles being more specifically linked to the disease. Frequency or severity of attacks did not correlate with the presence of vacuoles but those were more easily found in patients with long term disease [10]. Vacuoles and tubular aggregates are frequent changes in PP and therefore helpful for the diagnosis. Important myopathic findings in the muscle biopsy suggest a permanent myopathy which probably developes after severe crises or long term disease [7]. CONCLUSION The clinical diagnosis of AS is not always apparent and physicians should be aware of the possible physical, clinical manifestations and laboratory findings; because early diagnosis and treatment may reduce the risk of potentially lethal cardiac dysrrhytmias. 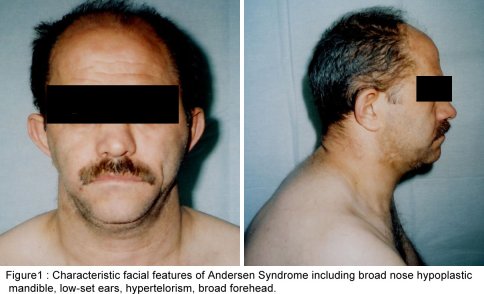 Figure 1 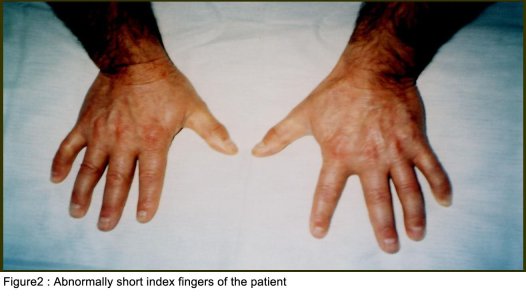 Figure 2  Figure 3 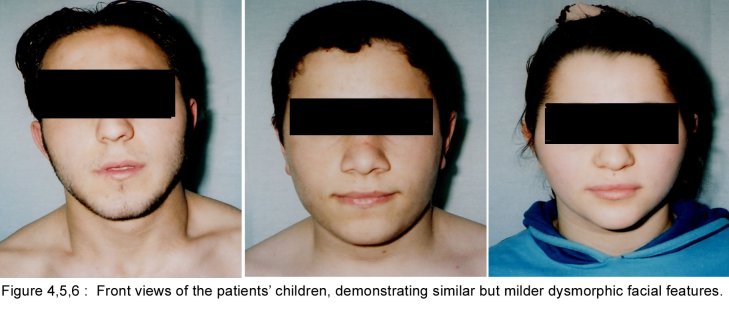 Figure 4 REFERENCES
|
© 2002-2018 African Journal of Neurological Sciences.
All rights reserved. Terms of use.
Tous droits réservés. Termes d'Utilisation.
ISSN: 1992-2647