
|
|||
|
EXPERIMENTAL STUDIES / ETUDES EXPERIMENTALES
D-CYCLOSERINE ENHANCES SPATIAL LEARNING PERFORMANCES OF RATS CHRONICALLY EXPOSED TO LEAD DURING THE DEVELOPMENTAL PERIOD.
LA D-CYCLOSÉRINE AMÉLIORE LES PERFORMANCES DE RATS AYANT ÉTÉ EXPOSÉS AU PLOMB DE MANIÈRE CHRONIQUE DURANT LE DÉVELOPPEMENT
E-Mail Contact - KAHLOULA Khaled :
bombixc2@yahoo.fr
ABSTRACT Chronic developmental lead (Pb) exposure has long been associated with cognitive dysfunction in children and animals. N-methyl-D-aspartate (NMDA) receptors, important in the synaptic mechanisms involved in learning and memory, are key target of lead toxicity. D-cycloserine (DCS), a partial agonist of the NMDA-associated glycine site, has been recognised as a potential cognitive enhancer. We investigated the potential effects of Pb exposure (lead acetate 0.2% through the drinking water) during gestation and lactation (GL), on the spatial learning and memory capacities of PN32 rats. We also evaluate the ability of DCS (30 mg/ml), administered daily 24h after weaning during 15 days, to attenuate Pb neurotoxicity-induced cognitive deficits. Results indicate that rats exposed to lead during gestation and lactation have a significantly increased latency to find the hidden platform and cover a significant longer distance compared to control-vehicle in the learning phase of the Morris water maze. However, the administration of DCS to GL animals improved significantly their learning performances compared with GL-vehicle. In contrast, there is no significant difference between all groups during the probe test and the visual cue test. Keywords: Neurotoxicity; Lead; Development; D-Cycloserine; Learning; Rat. RESUME Des études faites chez l’homme et l’animal montrent qu’une exposition chronique au plomb durant le développement provoque des altérations des fonctions cognitives. Les récepteurs au glutamate de type N-methyl-D-aspartate (NMDA), importants dans les mécanismes synaptiques impliqués dans l’apprentissage et la mémoire, sont les principales cibles du plomb. Il a été montré que le D-cyclosérine, un agoniste partiel du site glycine associé aux récepteurs NMDA, peut améliorer les fonctions cognitives. Nous avons étudié les effets possibles d’une exposition au plomb (acetate de plomb 0.2% dans l’eau de boisson) pendant la gestation et la lactation (GL), sur les performances d’apprentissage spatial et de mémorisation chez des rats âgés de 32 jours. Nous avons également évalué la capacité du D-cyclosérine (30 mg/ml), administré quotidiennement 24h après le sevrage durant 15 jours, à atténuer les déficits cognitifs induits par le plomb. Donc, l’amélioration de la fonction des récepteurs NMDA pourrait être une stratégie efficace pour améliorer les déficits d’apprentissage spatial associés à la neurotoxicité au plomb. Mots-clés: Neurotoxicité; Plomb; Développement; D-Cyclosérine; Apprentissage; Rat. 1. INTRODUCTION Lead (Pb) is a common environmental neurotoxic agent that is a major public health problem. Lead poisoning affects particularly the developing central nervous system [13, 24, 51]. Exposure to low levels of lead during early development is sufficient to cause long lasting behavioural abnormalities and cognitive deficits in human and experimental animals [38, 41, 59]. Pb-toxicity induces diminished IQ scores and reduced learning and memory capacities in children exposed early in life [5,7]. Rodents exposed to lead are impaired in cognitive tasks such as the step-down avoidance test, the contextual fear conditioning, the radial maze, the Morris water maze (MWM) [8, 28, 29, 40]. Lead alters glutamatergic function and also interferes with cholinergic and dopaminergic neurotransmission [6, 11, 33, 45]. This neurotoxicant impairs neurogenesis, synaptic plasticity, long-term potentiation (LTP) and cognitive function in experimental animals [19, 28, 55]. Several studies have indicated impairment in the induction, expression and maintenance of LTP in the hippocampus of animal exposed to lead during the early developmental period [2, 23, 20, 48]. The detrimental effects of lead on LTP and spatial learning have been attributed in one hand to the effect of lead on glutamate release [34, 35] and in an other hand, to the fact that lead is a potent inhibitor of N-methyl-D-aspartate (NMDA) receptors [18, 26]. Further, chronic exposure to lead alters the expression of NMDA subunit in the rat hippocampus [25, 43, 44], decreasing particularly the NR2A subunit expression, thus affecting NMDA complex composition, which is important in hippocampal development and maturation. Pb-exposition also leads to an alteration of downstream calcium signalling. Recent findings demonstrated that early lead exposure disrupts expression and phosphorylation of the cAMP-response element binding protein (CREB), a transcription factor important in synaptic plasticity and in learning and memory [56, 57]. Therefore, there is compelling experimental evidence that chronic lead exposure alters NMDA function in the rat hippocampus and this effects is associated with impairments in LTP and spatial learning [43, 55]. An improvement of performances in various learning tasks has been described after administration of D-cycloserine (DCS), a compound originally used as an antibiotic agent for the treatment of tuberculosis and later characterised as a partial agonist at the glycine modulatory site of the glutamatergic NMDA receptor [27, 58]. DCS is a cognitive enhancer in learning tests, such as the trace eye blink conditioning and the MWM task that are closely dependent on the hippocampus [36, 46, 54]. Moreover, DCS improves spatial performances of aged memory-deficient rats [3,4]. 2. MATERIAL AND METHODS 2.1 Animals and treatment Experiments were carried out on Wistar rats (obtained from Charles River) weighing 200‑350 g. Animals were housed with free access to water and food in an animal room with 12/12h light/dark cycle, at 22 ± 2 °C. They were mated one week after their arrival (three females and one male per cage). On pregnancy day 0 the dams were divided into 2 groups: one group received 0.2% lead acetate (Sigma-Aldrich Incorporated, France) in drinking distilled water during gestation and lactation (GL), whereas rats of the control group received distilled water without lead acetate (C), as described previously [45]. At birth, GL pups continued to receive lead acetate during lactation until postnatal day (PN) 21 and rats were weaned this day. Immediately after the behavioural evaluation, the rats were decapitated and blood samples were taken for Pb blood determination. Number and suffering of animals were minimised in accordance with the guidelines of the European Council Directive (86/609/EEC). 2.2 Drug preparation D-Cycloserine (DCS) was obtained from Sigma-Aldrich Incorporated (France). DCS was dissolved in distilled water and administered (i.p.) with a constant injection volume of 1 ml/kg. In order to maintain stability of the solution across days, DCS was frozen (‑20 °C) in 1 ml aliquots. Each day, DCS was thawed 15 min prior to injection. 2.3 Experimental design: chronic administration prior to assessment Animals were exposed to Pb during gestation and lactation (GL) and controls animals receive distilled water. In order to test the ability of DCS to attenuate Pb neurotoxicity induced cognitive deficits, the drug therapy was administered beginning 24 h after weaning. Randomly chosen animals of each group were injected (i.p.) with 30 mg/ml DCS (n=5; n=7, respectively) or distilled water (vehicle) (n=8; n=5, respectively). All animals were injected once daily on days 1-15 after weaning. During cognitive assessment (PN days 32-36) all subjects were given their injection 30 min prior to begin the MWM task. 2.4 Morris Water Maze Test The Morris water maze task was used to assess cognitive function, in particularly spatial learning and memory abilities [39]. In PN 32 rats, the test was conducted in a round white pool 90 cm in diameter with a 50 cm high wall. The pool was filled to a depth of 30 cm with water made opaque with white, non toxic water based paint. Water temperature was maintained at 21 ± 1 C° with an aquarium heater. The platform of 10 cm in diameter and 28 cm high (i.e., 2 cm below the water’s surface) was used as the hidden goal. The pool was located in a room with numerous extra-maze cues that remained constant throughout the experiment. Animals were tested on days 11-15 after weaning, with four trials per day for five consecutive days. On each trial, the animal was placed in the pool, facing the wall, at one of the four start locations (north (N), south (S), east (E), and west (W)). Start location sequences were randomly assigned each day prior to the start of testing. Rats were allowed to swim to the submerged platform placed in the NW quadrant during 60 s. If the platform was not found within the allowed time, the rat was manually placed onto it. Rats were allowed to rest on the platform for 20 s between each trial. A session of four trials was conducted each day from 9:00 to 11:00 A.M.. The experimenter was hidden from the view of the animals. The escape latency (time to find the platform) and path length (distance travelled to the hidden platform) were recorded by using a videotrack system (Viewpoint, France). A probe test was conducted the day following the end of the training session to further characterise swim task performance. In this test, the platform was removed and the rat was placed next to and facing the S side. Animals were allowed to swim freely for the original training session length of time (60 s) for a single trial. The time spent by animals in the previously correct quadrant (NW) was measured. Visual cue test was performed two hours after the end of the probe test in order to evaluate the visuo-motor abilities and the motivation of the animals. It was conducted by extending a large black flag above the water level from the submerged platform. The maximum time allowed was the same as the original training sessions. The escape latency and path length were measured during a single session of four trials. 2.5. Determination of blood lead level Blood samples were collected immediately after decapitation of animals in tubes pre-treated with 10 µl of heparin. The lead concentration in the blood was determined by using atomic absorption spectroscopy (model 800, Perkin Elmer). 2.6 Statistical analysis Results are expressed as mean ± standard error of the mean (SEM). Lead blood levels were compared between GL-vehicle and C-vehicle with a t-test. MWM data were analysed by two-way analyses of variance (ANOVAs), with the intoxication factor (GL or controls) and the treatment factor (DCS or vehicle), or a three-way ANOVA with day as the repeated measure. When a significant difference was found, the Student-Newman-Keuls post-hoc test was conducted. For all analyses, a difference was considered significant at p≤0.05. 3. RESULTS A student test reveals that lead concentration in blood of rats exposed to lead during gestation and lactation is significantly higher than the one of control rats [t(13)=11.78, p<0.001]. Lead concentrations in blood are 30.13 (±23.57) µg/dl for GL (n=8) and 0.30 (±0.61) µg/dl for control (n=7) rats. A three-way ANOVA with repeated measures comparing MWM cognitive performances during the training phase reveals a significant interaction between the treatment, intoxication and the repeated measure factors for latency to find the hidden platform, which means that animals perform differently according to their intoxication and treatment across days [F(3,63)=6.23, p<0.001]. An intoxication x treatment x day interaction also appears for distance crossed [F(3,63)=4.41, p<0.01]. A two-way ANOVA was conducted to compare the learning performances of rats day by day. There is no significant difference between all groups during the first and second days of training in terms of latency to find the hidden platform and swimming distance (p>0.05). On the third day of learning, assessment the ANOVA reveals a significant treatment effect for escape latency [F(1,21)=4.65, p<0.05] and distance crossed [F(1,21)=5.79, p<0.05]. Rats treated with DCS have significant better learning performances than control rats. A significant intoxication by treatment interaction is observed for the fourth day of training in terms of escape latency and path length ([F(1,21)=5.65, p<0.05], [F(1,21)=6.02, p<0.05] respectively). Post-hocs reveals that rats exposed to lead during gestation and lactation and treated with vehicle have a significant increased escape latency (p<0.01) and cover a significant longer distance (p<0.05) compared to control-vehicle. The administration of DCS to GL animals improves significantly their learning performances compared with GL-vehicle in terms of latency to find the hidden platform (p<0.01) and distance crossed (p<0.01). The performances of GL rats treated with DCS do not differ significantly from those of control-DCS rats (p>0.05). 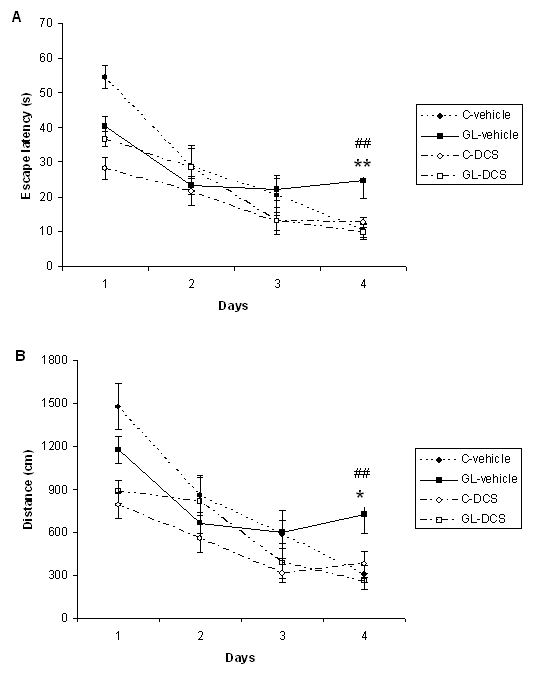 Figure 1 During the probe test, there is no significant difference between all groups for the time spent in the NW quadrant (p>0.05). 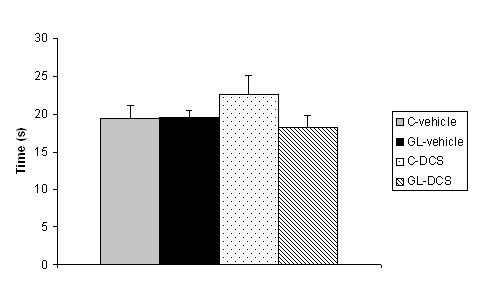 Figure 2 During the visual cue test, GL groups (treated with DCS or vehicle) do not present significantly different performances compared with control group in terms of escape latency and distance crossed (p>0.05). 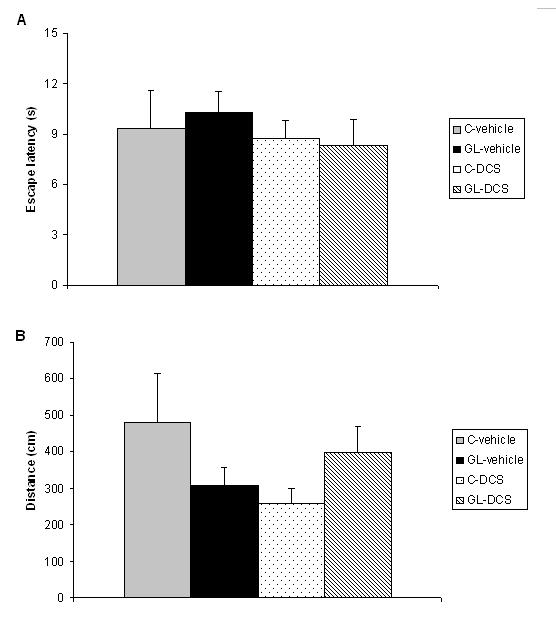 Figure 3 4. DISCUSSION The present study shows that, on one hand, a chronic lead intoxication of Wistar rats during gestation and lactation resulted in decreased learning performances in the MWM. On the other hand, the chronic administration of DCS at the dose of 30 mg/kg, on days 1-15 after weaning ameliorates significantly the cognitive impairments of rats exposed to lead until weaning. Several studies have revealed that Pb exposure produces neurological damage and behavioural disruptions in human beings and in experimental animals [1, 10, 21, 30, 32, 60]. The lowest Pb blood level at which behavioural abnormalities are detectable is 15-20 µg/dl in rodents and 10-15 µg/dl in children, as estimated by [12]. In our study, the blood lead level of animals exposed during the gestation and lactation is superior to the lowest Pb blood level at which are observed modification of behaviour and is close to the one described previously [8]. The glutamatergic system, specifically the NMDA receptor complex, is a key target site of lead neurotoxic action [13]. Lead exposure induces NMDA receptor functions changes [9], induces a wide variety of changes of intracellular signalling [15] and synaptic plasticity in hippocampus [22, 43] that are known to interfere with learning and memory. One pharmacological agent that has received attention as a promising cognitive-enhancer is the NMDA-glycine site partial agonist, D-cycloserine. Various studies indicate that DCS is an effective cognitive enhancer in multiple paradigms of cognitive impairment, including models of ageing and neurological lesions [4, 14, 50]. The aim of our work was to evaluate the possible effect of DCS in the case of a lead intoxication during development. Our results show that the learning deficits of rats exposed to lead during the gestation and lactation could be corrected by a chronic administration of DCS, as there is no significant difference between the performances of GL-DCS rats and controls rats in the learning phase of the MWM. 5. CONCLUSION In conclusion, the results from the present study show that learning deficits induced by a chronic Pb intoxication during gestation until weaning can be corrected by a chronic administration of DCS. Understanding the cellular action of DCS can help to find a strategy to prevent and treat the effect of Pb intoxication during development.
REFERENCES
|
© 2002-2018 African Journal of Neurological Sciences.
All rights reserved. Terms of use.
Tous droits réservés. Termes d'Utilisation.
ISSN: 1992-2647