
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
CLINICAL STUDIES / ETUDES CLINIQUES
SEX-RELATED DIFFERENCES IN STROKE OUTCOME AT THE UNIVERSITY OF MAIDUGURI TEACHING HOSPITAL, NORTHEASTERN NIGERIA
E-Mail Contact - MUSA Watila M. :
watilamusa@yahoo.com
Background: Studies have reported sex differences in stroke risk factor, presentation, morbidity and mortality. This study aims to determine the effect of sex on morbidity and 30-day fatality in patients with acute stroke. Methods: Ninety-one patients were recruited for the study. We documented sex differences in stroke presentation, stroke severity on admission and discharge, and 30-day in-hospital fatality. Continuous variables were assessed using the student t-test. While outcome measures were analysed using the logistic regression analysis. Conclusions: Sex differences in outcome exist in this study and women appear to have a poorer stroke outcome, more studies are needed to assess sex differences in response to therapy. Key words: blacks, outcome, sex, stroke. INTRODUCTION Stroke is a leading cause of long-term disability, (1) and the second most common cause of death worldwide, accounting for about 8% of total deaths in developing countries. (5) Men have a higher incidence of stroke, but women appear to have more severe strokes and a higher case fatality. (3) This has lead to an increasing concern for women with stroke with the Go red for women’ campaign, due to the increasing burden and uniqueness of stroke in women. (37, 8, 45, 13, 24, 10) Studies have reported sex-specific differences in stroke aetiology (37, 13, 24), presentations (45, 26, 44), diagnosis (39, 34, 47), treatments (13, 39, 36, 22) and outcomes (8, 13, 43, 19, 12). Reports have shown that women hospitalized for stroke were less likely to be investigated (34, 47), have a carotid endarterectomy (34), or receive thrombolytic therapy. (13, 36, 40) While some studies are in agreement that women have more severe strokes and a lower quality of life than men (39, 43, 24, 38), others are in disagreement. (19, 17, 35) Studies on sex difference in stroke mortality have been variable, some studies reported a higher mortality in women (24, 38, 4, 11), others reported a higher mortality in men (16, 42) and other studies reported no significant sex difference in stroke mortality (8, 19). These varying differences in mortality may be due to patient’s characteristics and how stroke and mortality are defined. (42) Reasons proposed for poorer outcomes in women include, older age and comorbidities; (24, 11) differences in acute stroke management; (8, 13, 34) and poorer functional recovery at rehabilitation. (33) Experimental studies are postulating cellular and pathophysiological basis for these sex differences, (25, 27) which is a subject for further researches. Based on the above observation we sought to determine sex differences in stroke morbidity and mortality, as such differences may provide an opportunity for improved patient care. MATERIALS AND METHODS The study cohort was 91 stroke patients admitted through the accident and emergency unit or the neurology clinic of the University of Maiduguri Teaching Hospital (UMTH), North-eastern Nigeria. Stroke was clinically defined by the WHO criteria as rapidly developing clinical sign of focal and/or global disturbance of cerebral function, with symptoms lasting twenty-four hours or longer or leading to death with no apparent cause other than of vascular origin. (50) During the five year period (2005-2009), two hundred and eighty-five (285) suspected stroke patients were attended to; one hundred and ninety-four patients were excluded from the study. Those excluded from the study were patients who had no computerized tomography (CT) or magnetic resonance (MRI) scan of the brain, those with a diagnosis of subarachnoid haemorrhage, subdural haematoma, those who died within 24hrs of admission and those with a past history of stroke. (Figure 1) All patients had an oral or written consent; consent was interpreted to some subjects into the local dialect for better understanding. History and examination was conducted and documented in the UMTH stroke proforma which included age and educational level. Risk factors for stroke such as hypertension (current blood pressure values 140/95 mmHg or features of long standing hypertension), atrial fibrillation (AF) (electrocardiographic evidence), transient ischaemic attack (TIA), diabetes mellitus (DM), smoking, alcohol consumption and a diagnosis of Human immunodeficiency virus (HIV) infection were recorded, this has been stated in an earlier study. (48) Medications taken by the patients before the stroke were also noted. Level of consciousness evaluated by the Glasgow Coma Score (a GCS < 8 is taken as coma). The distinction between ischaemic and haemorrhagic stroke was determined by CT or MRI. Patients with ischaemic stroke were categorized into; Total anterior circulation stroke (TACS), Partial anterior circulation stroke (PACS), Posterior circulation stroke (POCS) and Lacunar stroke. Stroke severity was assessed using the National Institutes of Health Stroke Scale (NIHSS). Disability and handicap were rated using the modified Rankin scale (mRS) and the Barthel ADL index (BI). These measures were also assessed at the time of discharge as outcome measures. The mRS identifies at a glance stroke disability, the BI assesses activity of daily living, but weighs heavily on motor functions, while the NIHSS provide the most prognostic information. (29) Duration of hospital stay was recorded only for those who survived till time of discharge. Death within 30 days attributable to the stroke was recorded from date of stroke admission. All the above data were categorized based on sex. Statistical Analysis Student’s t-test and the Z-test were used to assess continuous variables. The BI and the mRS were dichotomized for logistic regression analysis. The selected categories were severe stroke on admission (BI < 45 and mRS > 4), Poor ADL Status on discharge (BI < 45), independent on discharge (mRS <1 and BI > 90), and 30-day fatality was evaluated in a logistic regression analyses, controlling for age and clinical variables, these results were expressed as odds ratios (ORs). All statistical data were analyzed using SPSS (version 16.1) software. RESULTS During the five year period 285 patients had a diagnosis of suspected stroke, 194 patients were excluded from the study as shown in Figure 1. Ninety-one patients were registered for the study. All recruited patients were blacks residing in Nigeria. There were 61 men (67%) and 30 women (33%), mean age of 56.2 +11.1 and 55.6 + 12.4 years in men and women respectively. Compared to men, women were less likely to be formally educated (P = 0.024). Table 1 shows stroke subtypes. There was no statistical difference in stroke subtype; as 25 (83.3%) women compared with 48 (78.7%) men had CT evidence of infarction (P=0.812), while 5 (16.7%) women compared with 13 (21.3%) had haemorrhagic stroke (P = 0.812). Lacunar stroke was more common in men (P = 0.048), with no significant difference in other syndromes of ischaemic stroke. Men were more likely to take alcohol (P = 0.027) and to smoke (P = 0.046). There was no significant sex difference in other risk factors when comparing history of hypertension, DM, TIA, AF, HIV infection. Men were more likely to be treated with antihypertensives compared with women (P = 0.036) There was no significant difference in the use of diabetic, antiplatelet and lipid-lowering medications (Table 1). From the logistic regression in Table 2, women were about five times more likely to have severe stroke on admission (BI < 45, OR = 5.30; 95% CI, 1.10 to 25.62 and mRS > 4, OR = 5.38; 95% CI, 1.53 to 18.96), about four times likely to have a poor functional status on discharge ( BI < 45, OR = 4.40; 95% CI, 1.45 to 13.35) and twice as likely to die from stroke within 30 days of admission (OR = 2.19; 95% CI, 0.72 to 6.65). Urinary tract infection (UTI) was commoner in women (P = 0.023), with no major difference in other stroke complications (Figure 2). There was no sex difference in duration of in-hospital stay. (P = 0.168) Table 3, shows the summary of significant differences. DISCUSSIONS These data provide an evidence of differences by sex of stroke presentation and outcomes. This study shows that women were less likely to be educated, to smoke or to take alcohol. Women were however more likely to present in coma, and have to a more severe stroke at presentation. Women were also more likely to be disabled and to be handicapped on discharge. Despite the higher likelihood of men receiving antihypertensive medications prestroke, they still had a higher DBP on admission. This may be a reflection of the general lack of optimal BP control among hypertensives in the African subregion (7, 31). Although, there was no sex difference in hypertension as a risk factor in this study; studies have shown that women were more likely to be hypertensive, (22, 2, 51) though this may not be so in black Africans with stroke. In the study by Andersen et al (2), they reported that hypertension was slightly more prevalent in men below the age of 50 years, after which the prevalence increased in women. Since our patients are younger we may not have similar findings. We found no significant difference in risk factors such as DM, TIA and AF. Findings from other studies showed that women were more likely to have hypertension and AF (8, 13, 38, 11, 16), while men were more likely to have DM and heart disease (11, 16). Our finding of more frequent alcohol intake and smoking concurs with reports from other studies (8, 13, 11). Our study showed that men were more likely to be on antihypertensives prestroke, and no sex difference in the use of lipid lowering, antiplatelets or antidiabetic medications. Our observation is divergent to the studies showing that women were more likely to have treatment of high blood pressure prestroke (31, 9). The study by Smith et al (44) showed that women were more likely to be treated with antihypertensives, and less likely to receive antidiabetic, antithrombotic and lipid-lowering medications during the course of stroke treatment. The study by Khan et al, (22) is also in agreement that women were more likely to receive antihypertensive after a stroke. Report by Bushnell et al (6) on the use of statins showed that there was no sex difference in the use of statins or any statistical interaction between the effects of sex and statin use on the risk of stroke. In our study lacunar strokes were commoner in men; this is congruent with a study by Foster et al (10) and divergent to some studies that reported lacunar strokes occurring more frequently in women with TACS. (44, 39, 35) Women were reported to be older in several studies, (45, 13, 10, 44, 11) but this is not so in our study. The Framingham study (35) observed that women developed stroke an average of 5 years later than men. This difference in our study may be due to the relatively younger age of our cohort, smaller number of women participants and women in our community are less likely to seek medical attention early due to socioeconomic reasons, this may influence poorer outcomes and not reach the hospital alive. (32, 20, 49) The women in this study were less likely to be educated compared to men; it has been shown that level of education influences socioeconomic status. A lower socioeconomic status not only increases the likelihood of having a stroke (20), but also impacts negatively on stroke presentation and care. (52) Although other reports have shown no differences in stroke severity and case fatality rate (10, 17, 35); this study is in agreement with studies done elsewhere indicating that women were more likely have a severe stroke on admission and have worse outcomes after stroke, women were more likely to be disabled and handicapped on discharge, less likely to achieve activities of daily living independence and have a poorer quality of life. (8, 39, 43, 12, 24, 15) An excess of lacunar strokes in this study may contribute to the better outcome in men compared to women. A study by Sacco et al (41) observed that patients with lacunar stroke have a better outcome compared to those with nonlacunar strokes. In keeping with a more severe stroke women were more likely to be comatose at presentation compared to men, as reported by Gall et al (11). Gargano et al (14) showed no sex difference in patients presenting with coma, but asserted that delay in presentation to the emergency room may explain some of the differences in symptoms. Our study showed that women were twice as likely to die within thirty days from a stroke. This is consistent with some reports, (38, 11) but at variance with other studies. (8, 13, 10, 19) There are other studies that have shown lower rates of mortality in younger women than men and higher number in older women where the absolute burden of stroke is greater (24, 4). The higher mortality in women in other studies, were attributed to increasing age, a higher stroke severity and lower quality of care in women compared with men. (38, 4) There was no significant difference in the duration of hospital admission; this is congruent with a study by Zhu et al (53) who reported no sex difference in length of hospital stay. In our study UTI was commoner in women, and is in accordance with other studies. (13, 39) Studies have shown that women were less likely to be investigated or treated with thrombolytics, (8, 13, 39, 47), but it is not universal. It is interesting to note from studies by Kent et al (21) and Sacco et al (40) that women were more likely to benefit from thrombolytic therapy. This may be an area of further research in our environment. Gargano et al (13) in their study explaining why women were less likely to be treated argued that women were older than men and this influences management owing to comorbid conditions. Women are less likely to have surgical interventions like carotid endarterectomy. (34) A study by Tell et al (46) observed that men were more likely to have carotid atherosclerotic disease than women, and may explain why men have higher endarterectomies. Studies have postulated why women are likely to have severe stroke; Women brains are more likely to produce a stronger and more sustained inflammatory response compared to that of men in animal studies, consistent with increased immune responses (25, 27). In an experimental study with rats reported that oestrogen offers some neuroprotection in neuronal injury and this may be lost as women become older, (28) others have postulated the role of parity and increasing parity with increased mortality from both Ischaemic and haemorrhaghic stroke. (30, 18, 23) The small sample size is a limitation of this study. We did not consider sex differences in recognition of stroke symptoms; as this may influence presentation and subsequent care. We also did not consider sex related effect of stroke care. CONCLUSION This study reveals sex differences in stroke presentation and outcome among Nigerians. After adjusting for cofounding variables we observed that women have more severe stroke on admission, and were more likely to be disabled and handicapped on discharge. In keeping with a severe stroke, they were more likely to be in coma on admission. Women were twice more likely to die from a stroke. ACKNOWLEDGEMENTS We acknowledge the contributions of Dr. A. Ahidjo of the radiology department and Dr. AA Gadzama of the Chemical pathology department. 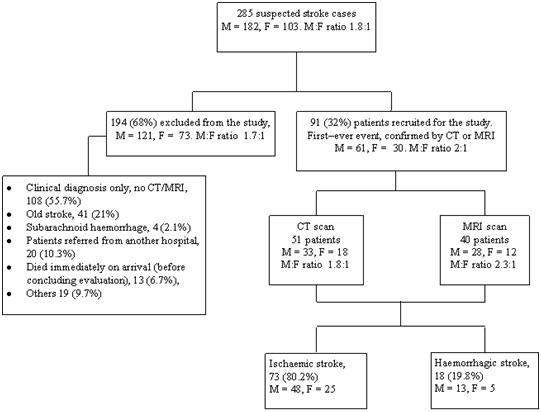 Figure 1. Inclusion and exclusion criteria, diagnosis and type of stroke. (48)
TABLE 1. COMPARISON OF DEMOGRAPHIC AND CLINICAL CHARACTERISTICS OF SUBJECTS BY SEX
TACS – total anterior circulation stroke, TABLE 2: INDEPENDENT PREDICTORS OF STROKE SEVERITY, DISABILITY AND 30-DAY MORTALITY AMONG FEMALE SEX.
Logistic regression analysis of variables, * P < 0.05 Table 3. Summary of significant differences
NIHSS – National Institutes of Health Stroke Scale. REFERENCES:
|
© 2002-2018 African Journal of Neurological Sciences.
All rights reserved. Terms of use.
Tous droits réservés. Termes d'Utilisation.
ISSN: 1992-2647
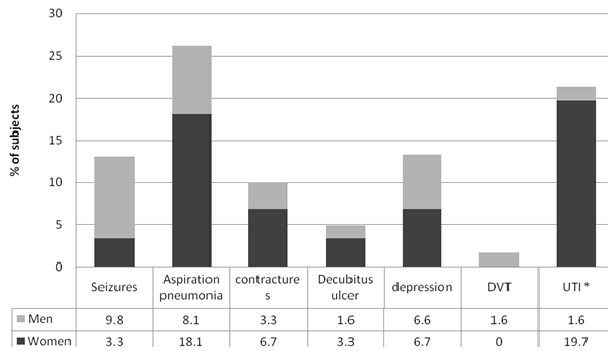 Figure 2. Stroke complications. Stacked bars are percentages of subjects with complications comparing sex groups. Asterisk * indicates P < 0.05.[/caption]
Figure 2. Stroke complications. Stacked bars are percentages of subjects with complications comparing sex groups. Asterisk * indicates P < 0.05.[/caption]