
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
REVIEW / MISE AU POINT
THE ROLE OF STATINS IN ALZHEIMER’S DISEASE: A META-ANALYSIS
E-Mail Contact - GIZACHEW Seyoum :
sweetseyoum@yahoo.com
SUMMARY Background In Alzheimer’s disease (AD) Amyloid Beta (Aβ) is deposited in the form of extracellular plaques and previous studies have showed Aβ generation is cholesterol dependent. The use of statins in the prevention and treatment of AD is poorly explored. The aim of this work was, therefore, to perform a review of studies on the efficacy and safety of statins in the prevention as well as treatment of AD. Methods and Findings Medline and Cochrane Database of Systematic Reviews search was performed for original research articles published in English language in which participants received any type of statins for at least 6 months and evaluated for their cognitive changes. Selected articles were grouped into two, randomized controlled trials (RCTs) and observational studies, and meta-analyzed separately. Thirteen studies identified, 4 RCTs including 1153 AD patients with trial period ranging from 26 to 72 weeks and 9 observational studies including 21,819 study participants with follow up period of up to 12 years. The 4 RCTs assessed change in Alzheimer’s Disease Assessment Scale- consisting of the cognitive subscale (ADAS-Cog) and when the results of each studies were combined there was no significant difference in ADAS-Cog between the statin and placebo group [Mean difference = -0.57, 95% CI, -1.39, 0.25, p=0.17]. Four of the 9 observational studies provided computed effect sizes in the form of Hazard ratio (HR) and common HR were computed and showed that statins had significant protective effect against AD [HR=0.69, 95% confidence interval (CI), 0.542, 0.882, p=0.003]. Three of the 9 observational studies were also combined for their Odds ratio (OR) and they showed that statins were protective against AD [OR=0.447, 95% CI, 0.299, 0.668, p=000]. Treatment related adverse effects were similar between statin and placebo [OR=2.84, 95% CI, 0.41, 19.69, p=0.29]. Conclusion Though observational studies have shown statins’ protective effect against AD, there is insufficient evidence to recommend statins for the treatment of AD, as RCTs failed to show significant efficacy. Statins are generally well tolerated in AD. Keywords: Alzheimer’s Disease; Meta-analysis; Prevention; Statins; Treatment BACKGROUND Alzheimer’s disease (AD) is an age-related neurodegenerative disease characterized by a progressive loss of memory associated with other cognitive sphere deficits interfering with social and occupational functioning (20). The global prevalence of AD was estimated at 26.55 million in 2006 (3). It has also been projected that worldwide prevalence will quadruple to 106.2 million, with 1 in 85 persons living with AD by the year 2050 (3). AD is the commonest type of dementia encountered in older Patients (12). Dementia causes a significant financial burden to society, worldwide societal costs estimated at $315 billion in 2005 ($105 billion were for informal care) (32).There are a number of non-pharmacological and symptomatic pharmacological approaches to treat AD (9). None of these, however, can prevent, cure or stop the progression of the disease (9). The brain is the most cholesterol-rich human organ (26). Hypercholesterolemia is one of the modifiable risk factors for AD (1, 23). A central event in the development of AD is thought to be abnormal processing of the cell membrane-associated amyloid precursor protein (APP) followed by deposition of toxic Aβ protein in the form of amyloid plaques in the extracellular space of the neocortex (7, 25). High cholesterol level may increase the activity of the β- or γ-secretase enzymes that generate Aβ from APP, and may decrease the flux of APP through the nonamyloidogenic α-secretase pathway (26, 24). Once Aβ has been produced, the cholesterol level could also influence its aggregation state (24). Several studies in cell culture and animals have demonstrated that treatment with cholesterol lowering drugs, such as statins, reduces the production of Aβ (28, 5). It was, therefore, hypothesized that reduction of Aβ levels by statins may have neuro-protective effects in patients with AD (33, 27). Cholesterol forms an essential component of cell membranes, and has a crucial role in the development and maintenance of neuronal plasticity and function (26). Statin induced reduction in cholesterol concentration in the central nervous system may, therefore, cause neuro-cognitive deficits (18, 19, 13). The aim of this work was, therefore, to review the studies on the efficacy and safety of statins in the prevention and treatment of Alzheimer’s disease as well as the role of baseline cholesterol level, Apolipoprotein (APO) E genotyping, age and cognitive level on the treatment outcome. METHODS Inclusion Criteria
Search Strategy A comprehensive systematic search for published articles and conference proceedings was undertaken with the electronic database Medline via PubMed, and Cochrane Database of Systematic Reviews, using the following combination of medical subject headings (MesH) terms or key words: “Statins” and “Alzheimer’s Disease”. The search was restricted to articles published in English language irrespective of the study place. Reference lists of identified articles were also searched. Data Extraction The summary statistics required for each trial and each outcome for continuous data were the mean change from baseline, the standard error of the mean change, and the number of patients for each treatment group at each assessment. Where changes from baseline were not reported, the mean, standard deviation and the number of patients for each treatment group at each time point was extracted. For binary data (commonly found in observational studies) computed effect sizes (OR, HR) and their corresponding confidence limits were sought. Data Analysis Outcome measures that were extracted from non-randomized studies analyzed separately from the outcome measures extracted from randomized trials to avoid bias (11). Randomized trials The outcomes measured in clinical trials of AD and cognitive impairment often arise from ordinal rating scales. Where the rating scales used in the trials have a reasonably large number of categories (more than 10), the data were treated as continuous outcomes arising from a normal distribution (17). Summary statistics (sample size (n), mean and standard deviation) were required for each rating scale at each assessment time for each treatment group in each trial for change from baseline. The mean change and standard deviation were calculated from the available data. All data extracted were then entered into Review Manager (RevMan), Version 5.1 (The Cochrane Collaboration, Oxford, England) for analysis. The duration of the trials varied from 26-72 weeks. A separate meta-analysis was conducted for each period. Some trials may contribute data to more than one time period if multiple assessments have been done. Observational studies For binary outcomes, such as progression of AD, severity of AD, the odds ratio, relative risk, or hazard ratio were used to measure treatment effect. The Comprehensive Meta-Analysis software (Biostat, Englewood, NJ, USA), Version 2 was used for data analysis. In all cases the overall estimate from a fixed effect model is presented. Presence of heterogeneity was assessed using the Cochran Q statistic, and I2 statistic was also used to quantify the degree of statistical heterogeneity. If there was significant heterogeneity a random effects model will be presented. A 2-sided alpha error of less than 0.05 was considered to be statistically significant (P<0.05). Potential publication bias was assessed by visual inspection of the funnel plot produced by plotting the standard error against the mean difference of RCTs or log OR (HR) of observational studies. RESULTS The flow of studies through the review process is outlined in Figure 1. After a literature search and selection based on the inclusion criteria as described in the methods, a total of thirteen studies were identified that met inclusion criteria. All the thirteen studies (nine observational studies and four randomized controlled trials) were meta-analyzed separately. Tables 1 and 2 show RCTs and observational studies with their characteristics, respectively. Of the 4 RCTs, 2 were undertaken in USA, 1 in Germany and the remaining one was an international study in which patients were recruited from 10 different countries. Six of the 9 observational studies were undertaken in USA and the remaining in Canada, France and the Netherlands. Overall, 1153 study subjects were included in the 4 RCTs with trial period ranging from 26 to 72 weeks. In the 9 of observational studies, a total of 21,819 study participants were included with follow-up period of up to 12 years. Randomized Controlled Trials The four studies assessed change in ADAS-Cog and as study periods varied between 26 to 72 weeks, separate meta-analysis was conducted for each period (table 3). When the four studies were combined there was no significant difference in ADAS-Cog between the statin group and placebo group (p=0.17) (table 3). As the Simons 2002 study was conducted for 26 weeks, data from ADCLT 2006, LEADe 2010, Sano 2011 and Simons 2002 at 6 months were combined and there was no significant difference in ADAS-Cog between the statin and placebo groups (p=0.49). As the ADCLT 2006 study was conducted for 12 months, data from ADCLT 2006, LEADe 2010 and Sano 2011 at 12 months were combined, and there was no significant difference in ADAS-Cog between the statin and placebo groups (p=0.60). Eighteen months data from LEADe 2010 and Sano 2011 on Change in ADAS-Cog were also combined using random effect model (due to presence of heterogeneity), and there was no significant difference in ADAS-Cog between the statin and placebo groups (p=0.86). Data on change in MMSE were also available from the four studies and separate meta-analysis was conducted for each period as it was done for ADAS-cog (table 4). No significant beneficial effect on MMSE was seen with statin treatment at any time. Three of the included RCTs (ADCLT 2006, LEADe 2010 and Sano 2011) also provided data on effect of statins on behavior using the instrument Neuropsychiatric Inventory Caregiver Distress Scale (NPI)) of AD patients. Data from these three studies were combined at the end point of each study and at 12 months (table 5). The effect was significantly different between groups only at 12 months (p=0.04) but not at end point (p=0.07) analysis. Eighteen months data from LEADe 2010 and Sano 2011 were also combined and found to be non significant (table 5). 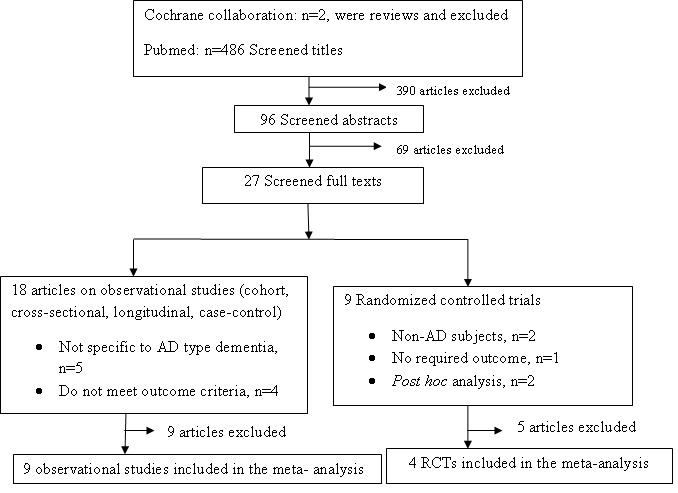 Fig. 1 Results of Pubmed and Cochrane collaboration database searches. Observational Studies One of the 9 included observational studies (i.e. Murali 2004) provided data using change in MMSE from baseline to follow-up period and the effect of statin on MMSE in statin users was not significant compared to statin non-users (p=0.429). Four of the remaining eight studies (Arvanitakis 2008, Higdon 2004, Li 2010, Rotterdam study 2009) provided computed effect sizes in the form of HR and the rest four (Hajjar 2002, Masse 2005, Peter 2005, Rockwood 2002) in the form of OR. The software used in the analysis (Comprehensive Meta-Analysis software) failed to accept data (OR, upper and lower limits) from Peter 2005 and reported that its upper and lower limit log values were not symmetric. Common HR computed from the above mentioned four studies showed that statin use had significant protective effect against AD [HR=0.69, 95% CI, 0.542, 0.882, p=0.003]. Computation of common OR from the above mentioned three studies also resulted in common OR=0.447, 95% CI, 0.299, 0.668, p=000, indicating that statin users had 0.447 times lower risk of AD compared to statin nonusers. Baseline cholesterol level, APOE genotyping, age and cognitive level and treatment outcome: Data provided in ADCLT 2006 revealed that patients who improved on the ADAS-Cog had higher baseline cholesterol levels than those who deteriorated. (Mean change in ADAS-Cog score -2.14 ±1.20 in atorvastatin + cholesterol >200mg/dl group, P=0.045 compared with placebo ≥200 mg/dl; 0.11±0.68 in atorvastatin + cholesterol <200mg/dl group). ADCLT 2006 also indicated that significant difference was seen in ADAS-Cog performance at 6 months between the atorvastatin and placebo groups in individuals with an APOE ε4 allele (p=0.012) but not between the groups comprised of subjects without an APOE ε4 allele (p=0.967). However, Arvanitakis 2008 and Peter 2005 mentioned that there was no interaction of statins with APOE ε4 allele. Rotterdam study 2009 provided data that the protective effect of statin use was similar for persons with an ApoE4 allele (adjusted HR 0.50; 95% CI 0.26 to 0.94) and for persons without an ApoE4 allele (adjusted HR 0.61; 95% CI 0.32 to 1.18). Data from Higdon 2004 and Li 2010 revealed that, though overall interaction term for statin use-by-APOE ε4 was not significant, statin exposure was associated with a significantly lower risk of AD in the subjects < 80 years old at entry who had at least one APOE-ε4 allele and these data were combined and significant difference was seen [HR=0.31, 95% CI, 0.207, 0.739, p=0.004]. Among subjects treated with atorvastatin in ADCLT 2006, those who had improved on the ADAS-Cog at 6 months had baseline MMSE scores 2 points higher than those who continued to deteriorate (21.93±0.85 compared to 19.83±1.10, p<0.06). Data provided in Rockwood 2002 indicated that the OR for those younger than 80 years was 0.26 (95% CI, 0.08, 0.88); while for those 80 years and older, it was 0.50 (95% CI, 0.13-1.88). The effect remains protective in those 80 years and older, the CI includes 1.0. Higdon 2004 and Li 2010 provided data on potential differential effect of statins in different age groups (<80 vs ≥80 years). Data from these two studies were combined and showed that statin use was associated with lower risk for probable AD in younger study subjects (HR=0.474, 95% CI, 0.298, 0.754, p=0.002), but not with study subjects ≥80 years (HR=1.464, 95% CI, 0.869, 2.467, p=0.152). Safety: LEADe 2010: There were 60 (19.1%) atorvastatin-treated and 69 (21.2%) placebo-treated patients who experienced serious adverse events (SAEs), 6 of whom in the atorvastatin group and 1 in the placebo group considered treatment related by the investigator or sponsor. There were 9 deaths (2.9%) in the atorvastatin group and 6 (1.8%) in the placebo group. The SAEs for atorvastatin group were hepatitis, acute renal failure/rhabdomyolysis/pancreatitis, abdominal pain/ nausea/chest discomfort, transaminases elevation, liver disorder and gastrointestinal haemorrhage. Simons 2002: 1 patient had muscle pain without elevation of creatine kinase, 1 patient was withdrawn because creatine kinase was elevated. No adverse effects were reported in the placebo group. Sano 2011: The groups did not differ in the number of subjects with SAEs (placebo group: 54/202 [26.7%]; treatment group: 56/204 [27.5%]; p = 0.91), the number of subjects with serious adverse events requiring hospitalization (placebo group: 46/202 [22.7%], active treatment group: 53/ 204 [25.9%]; p = 0.52), and the number of deaths (placebo group: 9/202 [4.5%], active group: 5/204 [2.5%]; p = 0.29). The most commonly occurring adverse events were falls, agitation, and anxiety. Data from LEADe 2010, Sano 2011 and Simons 2002 on treatment related adverse events requiring treatment discontinuation were combined and no significant difference between statin and placebo groups was seen [OR=2.84, 95% CI, 0.41, 19.69, p=0.29]. DISCUSSION In this systematic review and meta-analysis, both randomized controlled trials comparing statins use in the treatment of AD with matching placebo, and observational studies comparing statin users associated risk for AD (or cognitive decline) with matching non-users were reviewed and analyzed separately. Including non-randomized studies in reviews can be used to provide evidence of effects (benefit or harm) that cannot be adequately answered by reviews of randomized trials (11). To be included in the review, duration of the studies were expected to be at least six months. Six month was chosen as this was felt to be the minimum length of time required to be on treatment to allow a disease-modifying effect and before any cognitive benefit could be attained (17). This review provides inconsistent evidence between RCTs and observational studies. Mean change in ADAS-Cog and MMSE from baseline were an outcome in the four of the RCTs and there was no significant difference between the statin and the placebo groups. Mean change in NPI from baseline was also an outcome in the three of the included RCTs, i.e., ADCLT 2006, LEADe 2010 and Sano 2011, and significant difference between the statin and the placebo groups was seen only at 12 months study period, not at end points. These indicated that statins were not efficacious in the treatment of AD. A previous systematic review of RCTs assessed treatment of dementia or AD by statins (17). This was published before the Sano 2011 results were available. Three studies were identified ADCLT 2005, LEADe 2010 and Simons 2002 as identified in this review, and there was no statistically significant treatment effect of statins on AD which is in agreement with the present review. While, common effect sizes computed from observational studies (common HR and OR) indicated that statins had strong protective effect against AD, though one of the studies (Murali 2004) failed to show significant protective effect. But the weight of Murali 2004 in the review of observational studies was very small (compared only 11 statin users with 22 statin non-users). There was evidence from ADCLT 2006 that greater cognitive effect from the statin (atorvastatin) was seen in patients with higher cholesterol and higher MMSE at baseline. ADCLT 2006 and combined results from two of the observational studies (Higdon 2004 and Li 2010) provided evidence that greater cognitive and protective effects were seen in participants harboring an APOE ε4 allele. There was also evidence from observational studies that better protective effect against AD from statins was seen in those participants whose baseline age was younger than 80 years. The statins were well tolerated and incidence of adverse effects was low. The statin group did not have a significantly higher rate of adverse effects requiring discontinuation of treatment when the data from the three of the RCTs, i.e., LEADe 2010, Sano 2011 and Simons 2002, were combined. There was no evidence that statins were detrimental to cognition. The main strength of this review was inclusion of observational studies and their separate analysis to provide evidence on association of statin use with AD which was not elucidated in the review of RCTs only. Publication bias is a potential limitation when carrying out a review. This source of bias has been addressed by using funnel plot, and there was no evidence of publication bias as assessed by visual inspection of the funnel plot produced by plotting the standard error against the mean difference of RCTs or log OR (HR) of observational studies. In summary, observational studies have shown that statins have strong protective effect against AD, whereas statins use in RCTs showed no significant effect on AD. This implies that there is insufficient evidence to recommend statins for the treatment of AD, as the level of evidence from RCTs outweighs than from observational studies. Negative clinical outcomes from RCTs accompanied by promising positive outcomes from observational studies signal a need for development of better randomized controlled study designs. Available RCTs designed to be run for shorter study periods than observational studies and that may affect the outcomes. From ADCLT 2006 there was some evidence that atorvastatin treatment was more beneficial at six months in AD patients with higher MMSE and higher cholesterol levels at baseline. There was also evidence from ADCLT 2006 and observational studies that statin treatment was more beneficial in study participants harboring an APOE-ε4 allele. And observational studies also suggested that statin use was associated with lower risk for probable AD in younger study subjects (<80 years), but not with study subjects ≥80 years. From the present study, we recommend to use large scale RCTs to further assess the impact of treatment at an earlier stage of the disease process, effect of age, effect of APO E ε-4 allele and effect of baseline cholesterol level.
Table 1: Characteristics of included RCTs
LDL-C= Low density lipoprotein cholesterol, SD= standard deviation, MITT= modified intent to treat, ID= Identification Table 2: Characteristics of included observational studies
LLA= Lipid lowering agents Table 3. Change in cognition measured by change in ADAS-cog (error score). Mean difference obtained by subtracting weighted mean change in ADAS-cog score of placebo group from weighted mean change in ADAS-cog score of statin group.
Table 4. Change in cognition measured by change in MMSE (correct score). Mean difference obtained by subtracting weighted mean change in MMSE score of placebo group from weighted mean change in MMSE score of statin group.
Table 5. Effect of statin on behavior, measured in NPI change (error score). Mean difference obtained by subtracting weighted mean change in NPI score of placebo group from weighted mean change in NPI score of statin group.
*- Significant difference between statin and placebo groups REFERENCES
|
© 2002-2018 African Journal of Neurological Sciences.
All rights reserved. Terms of use.
Tous droits réservés. Termes d'Utilisation.
ISSN: 1992-2647