REVIEW / REVUE DE LA LITTÉRATURE
AUTOIMMUNE ENCEPHALITIS: A MISSED DIAGNOSTIC AND THERAPEUTIC OPPORTUNITY
ENCEPHALITE AUTO-IMMUNE : UNE OPPORTUNITE DIAGNOSTIQUE ET THERAPEUTIQUE MANQUEE.
- Department of Neurology, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
E-Mail Contact - ROOS Izanne :
ABSTRACT
Encephalitis is a common clinical problem affecting 5 cases per 100 000 population annually. After exclusion of the usual infective causes, a number of cases remain unexplained. It has been observed that many such cases have an autoimmune basis resulting in disruption of synaptic and ion channel function. This diagnosis should be suspected based on subacute onset, short term memory loss, altered mental status or psychiatric symptoms in combination with new focal neurological deficits, new onset seizures, CSF pleocytosis or MRI features suggestive of encephalitis. As this is a treatable condition, with a good prognosis if recognised early, it is important not to miss the diagnosis.
Key Words: Autoimmune encephalitis
BACKGROUND AND HISTORY
The term encephalitis refers to a rapidly progressive encephalopathy (usually in less than 6 weeks) as a result of brain inflammation and has an estimated annual incidence of 5 cases per 100 000 population in a UK cohort.[22, 72] This clinical scenario is quite rightly, in the first instance, considered to have an infectious cause as any delay in the diagnosis and treatment is associated with a high morbidity and mortality. For example, a more than two day delay in treatment initiation of Herpes simplex encephalitis results in a three-fold worse outcome at 6 months.[62] In approximately 50% of cases, the aetiology remains undetermined but with increased awareness many cases previously labelled as idiopathic encephalitis are now being identified to have an autoimmune origin.[47, 77] In view of the initial presentation, many of these patients are mistakenly thought to have a psychiatric disorder resulting in a delay in treatment.
The first description of paraneoplastic limbic encephalitis was in 1968 in association with lung, breast and thyroid malignancies.[9] These patients had paraneoplastic anti-Hu or anti-Ma2 antibodies: intracellular antibodies that result in irreversible, cytotoxic T cell responses with a poor prognosis and poor response to therapy. In 2005 Ances et al described 8 patients with limbic encephalitis who, at that time, had unidentified antibodies to neuronal surface proteins.[3] There has since been continued discovery of 1-2 such antibodies per year.[51] These antibodies reversibly disrupt synaptic functions and ion channels and are thus responsive to immunotherapy.[3] Unlike the intracellular neuronal antibodies, these neuronal cell surface antibodies have a more variable association with underlying malignancy (Table 1). In addition, autoimmune encephalitis affects patients of all age groups and detection has diagnostic, prognostic and therapeutic importance.
In this review, we discuss the clinical presentation of two of the most common autoimmune encephalopathies supported with case studies. We suggest a practical diagnostic approach to these patients.
Table 1: Autoimmune Encephalitis Syndromes
| |
Clinical Presentation |
Demographics/Age (median) |
Demographics/Gender |
Associated Malignancy/Frequency |
Associated Malignancy/Tumour type |
| Neuronal intracellular antibodies |
|
|
|
|
|
| Hu [1, 23] |
Limbic encephalitis* |
28-82 (63) |
75% male |
>95% |
SCLC |
| Ma2 [12] |
Limbic encephalitis* |
22-70 (34) |
68% male |
>95% |
Testicular |
| GAD [55] |
Limbic encephalitis*, stiff person syndrome |
>50 |
90% male |
25% |
Thymoma; SCLC |
| Neuronal cell surface antibodies |
|
|
|
|
|
| NMDAR [13, 52] |
Psychiatric, seizures, movement disorder, autonomic dysfunction, coma |
0.6-85 (21) |
80% female |
Varies with age and gender |
Ovarian teratoma (females 12-45y) |
| LGI1 [34, 43] |
Limbic encephalitis*, Faciobrachial dystonic seizures, Hyponatraemia |
30-80 (60) |
65% male |
5-10% |
Thymoma, thyroid, lung, renal cell |
| CASPR2 [34, 45, 71] |
Limbic encephalitis*, Morvan’s syndrome, Neuromyotonia |
46-77(60) |
90% male |
20-50% |
Thymoma |
| GABA A [60] |
Encephalitis, refractory seizures |
3-63 (22) |
83% male |
<5% |
Thymoma |
| GABA B [46] |
Limbic encephalitis*, seizures |
16-77 (62) |
50% male |
50% |
SCLC |
| AMPAR [42] |
Limbic encephalitis* |
38-87 (60) |
90% female |
70% |
SCLC, breast, thymoma |
| Glycine [8] |
PERM, stiff person syndrome, limbic encephalitis* |
1-75 (49) |
53% male |
10% |
Thymoma, lymphoma, breast |
| mGLuR5 [48] |
Encephalitis, myoclonus |
15-46 |
50% male |
70% |
Hodgkin’s Lymphoma |
| DPPX [5] |
Encephalitis, PERM, hyperekplexia |
15-76 |
65% male |
<10% |
Lymphoma |
| DR2 [11] |
Basal ganglia encephalitis, movement disorders |
1-15 (5) |
50% male |
Nil |
No association |
| IgLON5 [19] |
Sleep disorder, cognitive dysfunction, bulbar symptoms |
46-83 (64) |
54% female |
Nil |
No association |
*Limbic encephalitis: subacute confusion, memory disturbance, neuropsychiatric features and seizures.
Morvan’s syndrome: cognitive symptoms or seizures, peripheral nerve hyperexcitability and insomnia
Abbreviations: AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; CASPR2, contactin-associated protein-like 2; DR2, dopamine receptor 2; DPPX, dipeptidyl-peptidase-like protein-6; GABAR, gamma-amino-butyric acid receptor; GAD,glutamic acid decarboxylase; IgLON5, immunoglobulin-like family member 5; LGI1, leucine-rich, glioma-inactivated 1; mGluR5, metabotropic glutamate receptor 5; NMDAR, N-methyl-D-aspartic acid receptor; PERM, progressive encephalomyelitis with rigidity and myoclonus.
METHODS
Relevant articles were identified through PubMed searches of articles published in English up to 1 May 2017. Search terms included (alone or in combination) encephalitis, encephalopathy, limbic or autoimmune, which yielded 358 results. Additional articles were identified in references. Priority was given to large case series.
Written consent was obtained from patients described in the case reports.
Anti-NMDA Receptor Encephalitis
This disorder is associated with IgG antibodies against the GluN1 subunit of N-methyl D-aspartate (NMDA) receptors.[20] In vitro studies have shown that antibodies bind to NMDA receptors resulting in cross-linking and internalization. The degree of damage correlates with antibody titres and reverses with antibody removal.[31]
NMDA encephalitis has been found to be a common cause of encephalitis and the most common form of autoimmune encephalitis.[13] In an English series, 4% of patients with encephalitis were diagnosed with NMDA encephalitis.[21] The California encephalitis cohort identified NMDA encephalitis as being the most common cause of encephalitis, 4 times more common than viral causes, although this cohort was likely influenced by referral bias.[18] An intriguing finding is that in some patients herpes simplex encephalitis may also trigger other autoimmune reactions such as NMDA encephalitis.[15, 27]
The earliest reports of NMDA encephalitis were in 2005 and 2007 when Vitaliani and Dalmau described young women with ovarian teratomas presenting with psychiatric abnormalities, movement disorders and central hypoventilation.[14, 76] These descriptions resulted in rapid identification of increasing numbers of cases, with more than 600 cases being described in the literature by 2013.[68]
The clinical presentation and association with an underlying tumour varies with age and gender. The median age of presentation is 21 years but has ranged from 8 months to 81 years. Eighty one percent of the patients are female. Associated tumours are present in 38% of patients, predominantly in females between the ages of 12 and 45. Males are more likely to present below the age of 12 or over 45. Ninety seven percent of the associated tumours occur in females, 94% of which are ovarian teratomas. The remainder of tumours are extraovarian teratomas, lung, breast, testicular, ovarian, thymic and pancreatic in origin.[68]
These patients typically present in a step wise manner, although steps may occur in any order, all steps do not have to be present and seizures can occur at any stage of disease.
In 70% of patients a prodrome may precede the onset of other symptoms by up to 2 weeks. Prodromal symptoms include headache, fever, nausea, diarrhoea or features of an upper respiratory tract infection. Psychiatric symptoms occur next and range from anxiety and insomnia to mania, paranoia, hallucinations and grandiose delusions.[32, 63] In some patients psychiatric symptoms may predominate, resulting in psychiatric admission.[61] Atypical features for schizophrenia, such as pronounced catatonia or associated movement disorders and seizures, should therefore prompt psychiatrists to exclude an underlying autoimmune encephalitis.[70] Short term memory loss is common. Subsequent symptoms include movement disorders, autonomic dysfunction and central hypoventilation. The range of movement disorders is vast including chorea, dystonia, oro-lingual dyskinesias and opisthotonic posturing. The presence of autonomic dysfunction and central hypoventilation often necessitates ventilation and support in an intensive care setting.[32, 63]
Monosymptomatic presentations are very rare in all age groups but males more commonly present with partial seizures.[57, 73] Even though the presenting symptoms may vary by age, within 4 weeks the majority of patients have developed a similar cluster of symptomatology.[68]
Patients with long-term psychiatric diagnoses have on occasion been found to have NMDA receptor antibodies. The majority of these patients do not have other symptoms described in the syndrome. It is important to note in these patients have IgA or IgM and not IgG antibodies, or antibodies against the GluN2 subunit, and not GluN1 subunit that has been associated with NMDA encephalitis. [6, 28, 41] Four percent of patients have been described to have an overlapping, concurrent or sequential demyelinating syndrome, sometimes with associated Aquaporin 4 antibodies.[67]
With treatment 53% of patients improve within 4 weeks and 80% at 24 months. Symptoms often resolve in the reverse order in which they presented. First line treatment consists of tumour removal (if present), steroids, intravenous immunoglobulins and plasmapharesis. Up to 40% of patients do not respond to first line therapy. Second line therapy consists of rituximab or cyclophosphamide (individually or in combination). Approximately 80% of patients who fail first line therapy have a good outcome at 24 months after second line therapy. [68] It is important to keep in mind though that even after improvement of the acute episode there may be persistent deficits in memory, executive function and behavioural disturbances.
The condition is associated with a 7% mortality rate and relapses occur in 12-20%. Relapses may be separated from the initial event by months to years, consist of only part of the initial syndrome and are usually less severe than the initial episode.[17, 68] Relapses are also more likely to occur if the presenting episode was not treated or undertreated.[35] Although relapses are less common in those who were treated for an underlying tumour, in the case of a relapse patients should be re-assessed for presence of underlying contralateral or recurrent teratoma.[32]
Case Report: NMDA Encephalitis (in a box)
A previously well 19 year old woman was referred to the neurology unit for investigation of a change in her mental state. Five days prior to presentation she reported hearing unfamiliar voices while studying and feeling suspicious of others. Two days later she stopped responding to those around her and subsequently had two generalised tonic clonic seizures. There was no viral prodrome. On examination she was unresponsive to any stimuli and had prominent oromandibular dyskinesias, dystonia and opisthotonic posturing. She required intubation, ventilation and ICU admission. While in ICU she had episodes of supraventricular tachycardia. The patient was commenced on IVI acyclovir and investigated for an encephalitis. The FBC and metabolic panels were normal, HIV ELISA negative and RPR non-reactive. A lumbar puncture showed 8 lymphocytes, 0 polymorphonuclear cells, protein 0.16 g/l, glucose 4.4 mmol/l (plasma 7 mmol/l). The CSF FTA and cryptococcal latex agglutination test were negative, as were the PCR assays for HSV, VZV and CMV. NMDA receptor antibodies were negative in the serum but positive in CSF. The EEG was in keeping with an encephalopathic state with an extreme delta brush pattern. MRI brain was normal. Pelvic ultrasound did not show an underlying ovarian teratoma. The patient did not respond to first line therapy with IV methylprednisone, intravenous immunoglobulins or plasma exchange. She subsequently improved after 5 doses of IV cyclophosphamide. After a three-month admission to ICU she was stepped down to a general ward. She was discharged home two months later with continued outpatient rehabilitation. The patient was continued on oral prednisone and azathioprine and has continued to improve. One year later she is considering resuming her postgraduate studies.
Voltage-Gated Potassium Channel Antibodies
Voltage-gated potassium channels (VGKC) form part a multiprotein neuronal complex. Prior to 2010 antibodies to the Kv1.1 and Kv1.2 subunits of the Shaker family of VGKC were recognised in association with limbic encephalitis, faciobrachial dystonic seizures, Morvan syndrome and neuromyotonia.[33, 66, 74] Subsequently it has been identified that contactin-associated protein-2 (Caspr2) and leucine-rich, glioma inactivated 1 protein (LGI1) are the true target antigens, each with unique clinical presentations.[34, 43, 71] References to VGKC encephalitis prior to 2010 was most likely referring to an encephalitis secondary to LGI1 antibodies. Antibodies to other extracellular domains of the VGKC are not pathogenic and do not require treatment with immunotherapy.[49]
LGI1 Antibodies
LGI1 is a protein that interacts with the pre-synaptic ADAM23 and post-synaptic ADAM22 and AMPA receptors, forming a trans-synaptic complex. It is postulated that the antibodies result in reduced AMPA inhibitory behaviour, therefore resulting in neuronal hyperexcitability.[58]
LGI1 antibodies result in the second most common form of autoimmune encephalitis, after NMDA.[52] The disorder has a male predominance, with a 2:1 male:female ratio and a median age at presentation of 63 years.[34, 36] The typical presentation is that of faciobrachial dystonic seizures affecting the arm and ipsilateral face, which may precede the onset of limbic encephalitis by up to 26 days.[36] These seizures last about 3 seconds each and may occur a median of 50 times per day. Identification of the disorder at this stage is important as early treatment may prevent the rest of syndrome from developing and improves the prognosis.[38] Limbic encephalitis then develops, which consists of subacute onset confusion, short-term memory disturbance and personality changes.[34] Patients may then go on to develop generalised tonic clonic seizures, focal seizures with impaired awareness of temporal lobe origin or focal seizures with associated piloerection.[34, 74, 79] Presentation in non-convulsive status is not uncommon. As these seizures do not respond to anticonvulsants but to immunosuppressive therapy the identification of the disorder is very important.[36] A unique feature of LGI1 encephalitis is hyponatraemia, which occurs in 60-88% of cases. This is thought to be due to LGI1 antibodies binding to antidiuretic hormone-secreting neurones.[37] In the majority of patients, there is no tumour association. Prognostically, 71% of patients are seizure free with monthly IV MTP but only 33% had normalization of memory.[54] Rapid identification and treatment of this disorder is important as a delay in institution of immunotherapy has been associated hippocampal atrophy and poorer long-term memory outcome.[16] Therefore, despite patients with LGI1 encephalitis having a more rapid response to treatment than those with NMDA encephalitis it is likely that they have a poorer long-term recovery.[51] Reported mortality is 6% and relapses occur in 15%.[34, 43]
Caspr2 Antibodies
Disease associated with Caspr2 antibodies is more diverse, consisting of central and peripheral nervous system involvement. Limbic encephalitis (42%) and Morvan syndrome (29%) are the most frequent clinical syndromes and develop over an average of 4 months.[71] Morvan syndrome refers to a combination of cognitive symptoms or seizures, peripheral nerve hyperexcitability and insomnia.
This condition occurs predominantly in males with a median age of 66, 20% of which have associated thymomas.[37, 71, 75] Although these patients show a good response to immunotherapy relapses occur in 25%.[45, 71]
Case Report: LGI1 Encephalitis (in a box)
A 65 year old man with a background history of diabetes and hypertension presented in April 2014 with a first episode generalised tonic clonic seizure lasting 10 minutes. He had no further neurological complaints and a normal neurological examination. He also had features of a right middle lobe pneumonia and cholangitis. The serum Na was 117 mmol/L with a urinary Na of 75 mEq/L, urine osmolality of 536 mOsm/kg and serum osmolality of 269 mOsm/kg. This was interpreted as syndrome of inappropriate ADH on the basis of underlying septicaemia, complicated by a seizure. A CT brain was normal. The underlying septicaemia was treated and he was commenced on fludrocortisone tablets and water restriction.
The patient was readmitted in June with further seizures and persistent hyponatraemia. His EEG was in keeping with a mildly encephalopathic state. A lumbar puncture was performed and showed 0 polymorphs, 24 lymphocytes, protein 0.54 mg/dL, glucose 3.7 mmol/L (serum 6) and was negative for oligoclonal bands. The CSF FTA, HSV, VZV, mumps and enteroviruses were negative. He was discharged on a higher dose of sodium valproate.
In July he was admitted to ICU in status epilepticus necessitating administration of anaesthetic agents. As part of treatment of refractory status epilepticus he also received IVI methylprednisone, intravenous immunoglobulin and was discharged on 3 anticonvulsant agents. His MRI showed bilateral T2 and FLAIR medial temporal lobe and right frontal lobe hyperintensities. On review in August his wife reported events which consisted of head and eye turning to the right, twitching of the right side of his face as well as dystonic posturing of the right arm. There were 3 or more episodes per hour, each lasting a few seconds to a minute. His wife also reported a 4 month decline in his short-term memory and hypersomnolence. The patient was subsequently tested for LGI1 antibodies, which were positive in the serum. A CT chest and abdomen excluded underlying malignancy. The patient was commenced on oral prednisone at 60mg daily and azathioprine 150mg daily. There was subsequent resolution of his faciobrachial dystonic seizures, normalisation of serum Na and improvement in his mental state. Eighteen months later the patient remains well.
Diagnostic Evaluation
An encephalitis should be suspected in any patient presenting with combinations of subacute onset (in the last 3 months) of confusion, behavioural change, change in mood or seizures. Initially, a viral cause is presumed and treatment should be commenced pending results of initial investigations (Table 2).[72] It should be kept in mind that despite high assay sensitivity and specificity, CSF HSV PCR may be falsely negative if performed in the first 24 hours of the illness and should therefore be repeated if clinically suspected.[78]
Table 2: Initial Evaluation of a Patient Presenting with Encephalitis
| Serological |
| blood cultures |
| HIV ELISA |
| RPR |
| |
| Cerebrospinal Fluid Analysis |
| Opening pressure |
| Chemistry: protein, glucose (with paired plasma) |
| WCC |
| HSV PCR |
| VZV PCR |
| Enterovirus PCR |
| CMV PCR (if immunocompromised) |
| FTA-ABS/TPHA |
| Cryptococcal antigen |
| GeneXpert and TB culture |
| |
| Imaging |
| CT Brain or MRI if available |
| Chest x-ray |
| |
| Other |
| EEG |
The majority of patients with autoimmune encephalitis have an abnormal lumbar puncture, with the exception being patients with LGI1 antibodies (normal in 59%).[39, 51] The most common findings are pleocytosis of less than 100 cells per L in 80%, mild-moderate increase of CSF protein in 30% and the presence of oligoclonal bands in 60%.[51]
The EEG is abnormal in most patients, and usually shows slow frontal or temporal activity, with or without associated epileptiform discharges.[40] Although the EEG is a useful test to rule out alternative causes of encephalopathy the only antibody specific finding is that of the extreme delta brush pattern in 30% of patients with NMDA receptor encephalitis.[64]
Typical MRI findings include T2/FLAIR hyperintensities in one or both medial temporal lobes (Fig. 1). These findings are however not specific, and may also occur in herpes encephalitis and in seizures of temporal lobe onset.[50, 59] Similarly, a normal MRI doesn’t exclude the diagnosis, as it may be normal in 66% with NMDA encephalitis [68] and 46% with LGI1 encephalitis.[36] However, even in patients with normal appearing MRI scans, medial temporal lobe hypermetabolism has been identified on FDG-PET imaging.[4, 30]
Due to the overlap in symptomatology, antibody testing is available as a commercial panel that includes antibodies against Caspr2, LGI1, NMDA, AMPA and GABAb. Testing against other antibodies is only performed in research laboratories. Detection of CSF antibodies to cell surface antigens strongly supports the diagnosis whilst the significance of antibodies in serum only has less clear significance.[60] In a study looking at paired CSF and serum samples in NMDA encephalitis, up to 14% of cases were serum negative but CSF positive.
Findings in serum alone have been described in LGI1, CASPR2, GlyR and GABAa. These are most often unreproducible and have uncertain disease relevance, suggesting false positive results.[51] IgG antibodies are pathogenic but this cannot be generalised to IgA and IgM antibodies, which may be present in healthy people.[10]
Therefore, when testing for neuronal surface antibodies it is advisable to do so in both the CSF and serum for a number of reasons:
1. CSF analysis should be performed to exclude an infectious aetiology such as HSV.
2. Fourteen percent of patients with NMDA encephalitis have antibodies in the CSF only. Percentages in other encephalitides are unknown.[26]
3. Uncertainty arises regarding clinical applicability of results in settings where serum antibodies are positive but the CSF was not tested.
4. There is better correlation in clinical course/prognosis between titers in CSF than in serum. However, clinical correlation and not isolated monitoring of titers is advised as the antibodies may remain positive for years after clinical recovery.[2, 29]
As early treatment improves outcome,[3, 7, 74] there has been a recent shift in guidelines towards earlier recognition and treatment of autoimmune encephalitides.[25] These guidelines are less dependent on results of antibody tests, as these may be negative in 10%, may not be accessible in all instances or may take weeks to obtain.[24] Despite these limitations the identification of specific antibodies still has value as a prognostic tool, guides the search for associated tumours and provides a definitive diagnosis.
In cases with a strong clinical suspicion of autoimmune encephalitis repeat testing of both CSF and serum samples should be performed as the finding of delayed seropositivity has been described.[65] Therapy with intravenous immunoglobulin may make result in false negative results, but this finding is less common in the CSF than serum. It is however suggested that in cases where patients fulfil the criteria for probable autoimmune encephalitis, and other causes have been excluded, treatment should be offered regardless of antibody status.[25]
Possible autoimmune encephalitis should be suspected based on the presence of the following three criteria:[25] (put in a box)
1. Subacute onset (within the last 3 months) of short term memory loss, altered mental status (personality change, lethargy or altered level of consciousness) or psychiatric symptoms
2. At least one of:
a. new focal neurological deficits
b. new onset seizures
c. CSF pleocytosis (white cell count of more than 5 cells/mm3)
d. MRI features suggestive of encephalitis
3. Exclusion of other causes
Therefore in the presence of these features, and the exclusion of abnormalities in the initial evaluation, patients should be referred for further neurological workup and management.
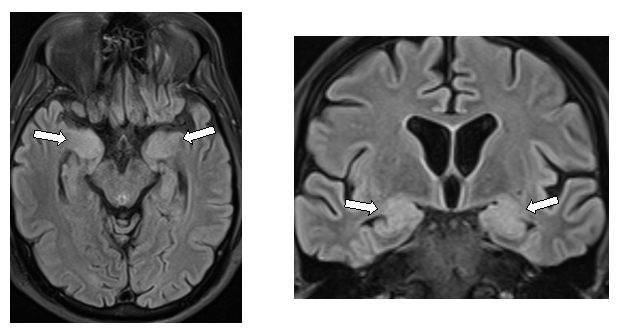 Figure 1: MRI findings in limbic encephalitis Treatment
Intracellular, paraneoplastic autoimmune encephalitides, with anti-Hu/Ma-2 or GAD antibodies are managed by treatment of the underlying malignancy. The response to therapy and prognosis in these patients is generally poor due to the irreversible T cell driven cytotoxicity.[44] In those with antibodies to cell surface antigens, however it has been established that early treatment with immunomodulatory therapy improves outcome, and may halt progression of the syndrome.[7, 36, 74] Published experience in treatment of NMDA and LGI1 encephalitis has largely determined the treatment approach in these patients.[34, 43, 68] There are however no specific guidelines for the treatment of autoimmune encephalitis, nor therapies that specifically target antibodies. Based on experience first line therapy consists of tumour removal (if indicated), steroids, intravenous immunoglobulins or plasma exchange. Patients who have a tumour have a worse outcome and more relapses if not resected.[32] If there is no response within 4 weeks therapy may be escalated to rituximab or cyclophosphamide. There has been a recent move towards the earlier use (or first line use) of second line therapy as this may result in a lower relapse rate with good tolerability.[68] The rationale is that these agents target antibody production by plasmablasts rather than neutralising or eliminating already formed systemic antibodies.[53] It is currently unknown how long treatment should be continued for patients with these disorders, thus individualised patient care decisions should be made.
Ongoing multidisciplinary care is of utmost importance in these patients and team members will vary during the disease course. It is important to note that these are disorders requiring high degrees of therapeutic commitment as average length of hospital admission is 3 months (NMDA encephalitis) with a longer period of rehabilitation thereafter.[51] It is therefore necessary to assemble a personalised team of specialists potentially consisting of neurologists, intensivists, neuropsychologists, psychiatrists and rehabilitation personnel in order to optimise patient outcome.[56]
Tumour Screening
Unlike encephalitides with antibodies against intracellular proteins (anti-Hu/Ma2), the association with an underlying malignancy is variable in patients with neuronal cell surface antibodies. The need for tumour screening should therefore be guided by the identified antibody together with the neurological syndrome, age and gender of the patient (see table 1). Syndromes with a low likelihood of associated malignancy may therefore not justify ongoing evaluation. In cases with high tumour associations however, if initial screening is negative repeat this should be repeated after 3-6 months and then 6 monthly for up to 4 years. Appropriate screening modalities for the thorax are a CT chest followed by FDG/PET, breast screening with a mammogram and screening for ovarian tumours with a transvaginal ultrasound followed by a CT pelvis. Screening with tumour markers alone is not reliable. [69]
CONCLUSION
With increased awareness autoimmune encephalitis has become one of the most frequent causes of encephalitis. This is a treatable condition affecting all age groups, and at times preferentially young individuals. Despite the poor prognosis in cases where diagnosis is delayed, with treatment a good outcome can be expected in 70-80% of individuals.[47] Thus, an increased awareness with prompt identification and treatment of these patients is paramount.
Acknowledgements
Dr B Bhagwan and Dr S Marais kindly allowed us to use the case reports of their patients with LGI1 and NMDA receptor encephalitides respectively.
Conflict of Interest
None
Author Contributions
AIB conceived the design and undertook critical analysis of the paper. IR participated in concept design, undertook the literature search and wrote the initial draft of the paper. The final manuscript was approved by both authors.
Funding sources
None
REFERENCES
- ALAMOWITCH S, GRAUS F, UCHUYA M, RENE R, BESCANSA E, DELATTRE JY. Limbic encephalitis and small cell lung cancer. Clinical and immunological features. Brain. 1997;120 ( Pt 6)(6):923-8.
- ALEXOPOULOS H, KOSMIDIS ML, DALMAU J, DALAKAS MC. Paraneoplastic anti-NMDAR encephalitis: long term follow-up reveals persistent serum antibodies. J Neurol. 2011;258(8):1568-70.
- ANCES BM, VITALIANI R, TAYLOR RA, LIEBESKIND DS, VOLOSCHIN A, HOUGHTON DJ, GALETTA SL, DICHTER M, ALAVI A, ROSENFELD MR, DALMAU J. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128(Pt 8):1764-77.
- BAUMGARTNER A, RAUER S, MADER I, MEYER PT. Cerebral FDG-PET and MRI findings in autoimmune limbic encephalitis: correlation with autoantibody types. J Neurol. 2013;260(11):2744-53.
- BORONAT A, GELFAND JM, GRESA-ARRIBAS N, JEONG HY, WALSH M, ROBERTS K, MARTINEZ-HERNANDEZ E, ROSENFELD MR, BALICE-GORDON R, GRAUS F, RUDY B, DALMAU J. Encephalitis and antibodies to dipeptidyl-peptidase-like protein-6, a subunit of Kv4.2 potassium channels. Ann Neurol. 2013;73(1):120-8.
- BUSSE S, BUSSE M, BRIX B, PROBST C, GENZ A, BOGERTS B, STOECKER W, STEINER J. Seroprevalence of N-methyl-D-aspartate glutamate receptor (NMDA-R) autoantibodies in aging subjects without neuropsychiatric disorders and in dementia patients. Eur Arch Psychiatry Clin Neurosci. 2014;264(6):545-50.
- BYRNE S, WALSH C, HACOHEN Y, MUSCAL E, JANKOVIC J, STOCCO A, DALE RC, VINCENT A, LIM M, KING M. Earlier treatment of NMDAR antibody encephalitis in children results in a better outcome. Neurol Neuroimmunol Neuroinflamm. 2015;2(4):e130.
- CARVAJAL-GONZALEZ A, LEITE MI, WATERS P, WOODHALL M, COUTINHO E, BALINT B, LANG B, PETTINGILL P, CARR A, SHEERIN UM, PRESS R, LUNN MP, LIM M, MADDISON P, MEINCK HM, VANDENBERGHE W, VINCENT A. Glycine receptor antibodies in PERM and related syndromes: characteristics, clinical features and outcomes. Brain. 2014;137(Pt 8):2178-92.
- CORSELLIS JA, GOLDBERG GJ, NORTON AR. « Limbic encephalitis » and its association with carcinoma. Brain. 1968;91(3):481-96.
- DAHM L, OTT C, STEINER J, STEPNIAK B, TEEGEN B, SASCHENBRECKER S, HAMMER C, BOROWSKI K, BEGEMANN M, LEMKE S, RENTZSCH K, PROBST C, MARTENS H, WIENANDS J, SPALLETTA G, WEISSENBORN K, STOCKER W, EHRENREICH H. Seroprevalence of autoantibodies against brain antigens in health and disease. Ann Neurol. 2014;76(1):82-94.
- DALE RC, MERHEB V, PILLAI S, WANG D, CANTRILL L, MURPHY TK, BEN-PAZI H, VARADKAR S, AUMANN TD, HORNE MK, CHURCH AJ, FATH T, BRILOT F. Antibodies to surface dopamine-2 receptor in autoimmune movement and psychiatric disorders. Brain. 2012;135(Pt 11):3453-68.
- DALMAU J, GRAUS F, VILLAREJO A, POSNER JB, BLUMENTHAL D, THIESSEN B, SAIZ A, MENESES P, ROSENFELD MR. Clinical analysis of anti-Ma2-associated encephalitis. Brain. 2004;127(Pt 8):1831-44.
- DALMAU J, LANCASTER E, MARTINEZ-HERNANDEZ E, ROSENFELD MR, BALICE-GORDON R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10(1):63-74.
- DALMAU J, TUZUN E, WU HY, MASJUAN J, ROSSI JE, VOLOSCHIN A, BAEHRING JM, SHIMAZAKI H, KOIDE R, KING D, MASON W, SANSING LH, DICHTER MA, ROSENFELD MR, LYNCH DR. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25-36.
- DESENA A, GRAVES D, WARNACK W, GREENBERG BM. Herpes simplex encephalitis as a potential cause of anti-N-methyl-D-aspartate receptor antibody encephalitis: report of 2 cases. JAMA Neurol. 2014;71(3):344-6.
- FINKE C, PRUSS H, HEINE J, REUTER S, KOPP UA, WEGNER F, THEN BERGH F, KOCH S, JANSEN O, MUNTE T, DEUSCHL G, RUPRECHT K, STOCKER W, WANDINGER KP, PAUL F, BARTSCH T. Evaluation of Cognitive Deficits and Structural Hippocampal Damage in Encephalitis With Leucine-Rich, Glioma-Inactivated 1 Antibodies. JAMA Neurol. 2017;74(1):50-59.
- GABILONDO I, SAIZ A, GALAN L, GONZALEZ V, JADRAQUE R, SABATER L, SANS A, SEMPERE A, VELA A, VILLALOBOS F, VINALS M, VILLOSLADA P, GRAUS F. Analysis of relapses in anti-NMDAR encephalitis. Neurology. 2011;77(10):996-9.
- GABLE MS, SHERIFF H, DALMAU J, TILLEY DH, GLASER CA. The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. Clin Infect Dis. 2012;54(7):899-904.
- GAIG C, GRAUS F, COMPTA Y, HOGL B, BATALLER L, BRUGGEMANN N, GIORDANA C, HEIDBREDER A, KOTSCHET K, LEWERENZ J, MACHER S, MARTI MJ, MONTOJO T, PEREZ-PEREZ J, PUERTAS I, SEITZ C, SIMABUKURO M, TELLEZ N, WANDINGER KP, IRANZO A, ERCILLA G, SABATER L, SANTAMARIA J, DALMAU J. Clinical manifestations of the anti-IgLON5 disease. Neurology. 2017;88(18):1736-1743.
- GLEICHMAN AJ, SPRUCE LA, DALMAU J, SEEHOLZER SH, LYNCH DR. Anti-NMDA receptor encephalitis antibody binding is dependent on amino acid identity of a small region within the GluN1 amino terminal domain. J Neurosci. 2012;32(32):11082-94.
- GRANEROD J, AMBROSE HE, DAVIES NWS, CLEWLEY JP, WALSH AL, MORGAN D, CUNNINGHAM R, ZUCKERMAN M, MUTTON KJ, SOLOMON T, WARD KN, LUNN MPT, IRANI SR, VINCENT A, BROWN DWG, CROWCROFT NS, HPA UHPA. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infectious Diseases. 2010;10(12):835-844.
- GRANEROD J, COUSENS S, DAVIES NW, CROWCROFT NS, THOMAS SL. New estimates of incidence of encephalitis in England. Emerg Infect Dis. 2013;19(9).
- GRAUS F, KEIME-GUIBERT F, RENE R, BENYAHIA B, RIBALTA T, ASCASO C, ESCARAMIS G, DELATTRE JY. Anti-Hu-associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain. 2001;124(Pt 6):1138-48.
- GRAUS F, SAIZ A, LAI M, BRUNA J, LOPEZ F, SABATER L, BLANCO Y, REY MJ, RIBALTA T, DALMAU J. Neuronal surface antigen antibodies in limbic encephalitis: clinical-immunologic associations. Neurology. 2008;71(12):930-6.
- GRAUS F, TITULAER MJ, BALU R, BENSELER S, BIEN CG, CELLUCCI T, CORTESE I, DALE RC, GELFAND JM, GESCHWIND M, GLASER CA, HONNORAT J, HOFTBERGER R, IIZUKA T, IRANI SR, LANCASTER E, LEYPOLDT F, PRUSS H, RAE-GRANT A, REINDL M, ROSENFELD MR, ROSTASY K, SAIZ A, VENKATESAN A, VINCENT A, WANDINGER KP, WATERS P, DALMAU J. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404.
- GRESA-ARRIBAS N, TITULAER MJ, TORRENTS A, AGUILAR E, MCCRACKEN L, LEYPOLDT F, GLEICHMAN AJ, BALICE-GORDON R, ROSENFELD MR, LYNCH D, GRAUS F, DALMAU J. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. The Lancet Neurology. 2014;13(2):167-177.
- HACOHEN Y, DEIVA K, PETTINGILL P, WATERS P, SIDDIQUI A, CHRETIEN P, MENSON E, LIN JP, TARDIEU M, VINCENT A, LIM MJ. N-methyl-D-aspartate receptor antibodies in post-herpes simplex virus encephalitis neurological relapse. Mov Disord. 2014;29(1):90-6.
- HAMMER C, STEPNIAK B, SCHNEIDER A, PAPIOL S, TANTRA M, BEGEMANN M, SIREN AL, PARDO LA, SPERLING S, MOHD JOFRRY S, GURVICH A, JENSEN N, OSTMEIER K, LUHDER F, PROBST C, MARTENS H, GILLIS M, SAHER G, ASSOGNA F, SPALLETTA G, STOCKER W, SCHULZ TF, NAVE KA, EHRENREICH H. Neuropsychiatric disease relevance of circulating anti-NMDA receptor autoantibodies depends on blood-brain barrier integrity. Mol Psychiatry. 2014;19(10):1143-9.
- HANSEN HC, KLINGBEIL C, DALMAU J, LI W, WEISSBRICH B, WANDINGER KP. Persistent intrathecal antibody synthesis 15 years after recovering from anti-N-methyl-D-aspartate receptor encephalitis. JAMA Neurol. 2013;70(1):117-9.
- HEINE J, PRUSS H, BARTSCH T, PLONER CJ, PAUL F, FINKE C. Imaging of autoimmune encephalitis–Relevance for clinical practice and hippocampal function. Neuroscience. 2015;309:68-83.
- HUGHES EG, PENG X, GLEICHMAN AJ, LAI M, ZHOU L, TSOU R, PARSONS TD, LYNCH DR, DALMAU J, BALICE-GORDON RJ. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010;30(17):5866-75.
- IIZUKA T, SAKAI F, IDE T, MONZEN T, YOSHII S, IIGAYA M, SUZUKI K, LYNCH DR, SUZUKI N, HATA T, DALMAU J. Anti-NMDA receptor encephalitis in Japan: long-term outcome without tumor removal. Neurology. 2008;70(7):504-11.
- IRANI S, LANG B. Autoantibody-mediated disorders of the central nervous system. Autoimmunity. 2008;41(1):55-65.
- IRANI SR, ALEXANDER S, WATERS P, KLEOPA KA, PETTINGILL P, ZULIANI L, PELES E, BUCKLEY C, LANG B, VINCENT A. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain. 2010;133(9):2734-48.
- IRANI SR, BERA K, WATERS P, ZULIANI L, MAXWELL S, ZANDI MS, FRIESE MA, GALEA I, KULLMANN DM, BEESON D, LANG B, BIEN CG, VINCENT A. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain. 2010;133(Pt 6):1655-67.
- IRANI SR, MICHELL AW, LANG B, PETTINGILL P, WATERS P, JOHNSON MR, SCHOTT JM, ARMSTRONG RJ, A SZ, BLEASEL A, SOMERVILLE ER, SMITH SM, VINCENT A. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol. 2011;69(5):892-900.
- IRANI SR, PETTINGILL P, KLEOPA KA, SCHIZA N, WATERS P, MAZIA C, ZULIANI L, WATANABE O, LANG B, BUCKLEY C, VINCENT A. Morvan syndrome: clinical and serological observations in 29 cases. Ann Neurol. 2012;72(2):241-55.
- IRANI SR, STAGG CJ, SCHOTT JM, ROSENTHAL CR, SCHNEIDER SA, PETTINGILL P, PETTINGILL R, WATERS P, THOMAS A, VOETS NL, CARDOSO MJ, CASH DM, MANNING EN, LANG B, SMITH SJ, VINCENT A, JOHNSON MR. Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain. 2013;136(Pt 10):3151-62.
- JARIUS S, HOFFMANN L, CLOVER L, VINCENT A, VOLTZ R. CSF findings in patients with voltage gated potassium channel antibody associated limbic encephalitis. J Neurol Sci. 2008;268(1-2):74-7.
- KAPLAN PW, SUTTER R. Electroencephalography of autoimmune limbic encephalopathy. J Clin Neurophysiol. 2013;30(5):490-504.
- KAYSER MS, TITULAER MJ, GRESA-ARRIBAS N, DALMAU J. Frequency and characteristics of isolated psychiatric episodes in anti-N-methyl-d-aspartate receptor encephalitis. JAMA Neurol. 2013;70(9):1133-9.
- LAI M, HUGHES EG, PENG X, ZHOU L, GLEICHMAN AJ, SHU H, MATA S, KREMENS D, VITALIANI R, GESCHWIND MD, BATALLER L, KALB RG, DAVIS R, GRAUS F, LYNCH DR, BALICE-GORDON R, DALMAU J. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol. 2009;65(4):424-34.
- LAI M, HUIJBERS MG, LANCASTER E, GRAUS F, BATALLER L, BALICE-GORDON R, COWELL JK, DALMAU J. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. 2010;9(8):776-85.
- LANCASTER E. Paraneoplastic disorders. Continuum (Minneap Minn). 2015;21(2 Neuro-oncology):452-75.
- LANCASTER E, HUIJBERS MG, BAR V, BORONAT A, WONG A, MARTINEZ-HERNANDEZ E, WILSON C, JACOBS D, LAI M, WALKER RW, GRAUS F, BATALLER L, ILLA I, MARKX S, STRAUSS KA, PELES E, SCHERER SS, DALMAU J. Investigations of caspr2, an autoantigen of encephalitis and neuromyotonia. Ann Neurol. 2011;69(2):303-11.
- LANCASTER E, LAI M, PENG X, HUGHES E, CONSTANTINESCU R, RAIZER J, FRIEDMAN D, SKEEN MB, GRISOLD W, KIMURA A, OHTA K, IIZUKA T, GUZMAN M, GRAUS F, MOSS SJ, BALICE-GORDON R, DALMAU J. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010;9(1):67-76.
- LANCASTER E, MARTINEZ-HERNANDEZ E, DALMAU J. Encephalitis and antibodies to synaptic and neuronal cell surface proteins. Neurology. 2011;77(2):179-89.
- LANCASTER E, MARTINEZ-HERNANDEZ E, TITULAER MJ, BOULOS M, WEAVER S, ANTOINE JC, LIEBERS E, KORNBLUM C, BIEN CG, HONNORAT J, WONG S, XU J, CONTRACTOR A, BALICE-GORDON R, DALMAU J. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology. 2011;77(18):1698-701.
- LANG B, MAKUCH M, MOLONEY T, DETTMANN I, MINDORF S, PROBST C, STOECKER W, BUCKLEY C, NEWTON CR, LEITE MI, MADDISON P, KOMOROWSKI L, ADCOCK J, VINCENT A, WATERS P, IRANI SR. Intracellular and non-neuronal targets of voltage-gated potassium channel complex antibodies. J Neurol Neurosurg Psychiatry. 2017;88(4):353-361.
- LANSBERG MG, O’BRIEN MW, NORBASH AM, MOSELEY ME, MORRELL M, ALBERS GW. MRI abnormalities associated with partial status epilepticus. Neurology. 1999;52(5):1021-7.
- LEYPOLDT F, ARMANGUE T, DALMAU J. Autoimmune encephalopathies. Ann N Y Acad Sci. 2015;1338:94-114.
- LEYPOLDT F, WANDINGER KP, BIEN CG, DALMAU J. Autoimmune Encephalitis. Eur Neurol Rev. 2013;8(1):31-37.
- LINNOILA JJ, ROSENFELD MR, DALMAU J. Neuronal surface antibody-mediated autoimmune encephalitis. Semin Neurol. 2014;34(4):458-66.
- MALTER MP, FRISCH C, SCHOENE-BAKE JC, HELMSTAEDTER C, WANDINGER KP, STOECKER W, URBACH H, SURGES R, ELGER CE, VINCENT AV, BIEN CG. Outcome of limbic encephalitis with VGKC-complex antibodies: relation to antigenic specificity. J Neurol. 2014;261(9):1695-705.
- MALTER MP, HELMSTAEDTER C, URBACH H, VINCENT A, BIEN CG. Antibodies to glutamic acid decarboxylase define a form of limbic encephalitis. Ann Neurol. 2010;67(4):470-8.
- MANN A, MACHADO NM, LIU N, MAZIN AH, SILVER K, AFZAL KI. A multidisciplinary approach to the treatment of anti-NMDA-receptor antibody encephalitis: a case and review of the literature. J Neuropsychiatry Clin Neurosci. 2012;24(2):247-54.
- NIEHUSMANN P, DALMAU J, RUDLOWSKI C, VINCENT A, ELGER CE, ROSSI JE, BIEN CG. Diagnostic value of N-methyl-D-aspartate receptor antibodies in women with new-onset epilepsy. Arch Neurol. 2009;66(4):458-64.
- OHKAWA T, FUKATA Y, YAMASAKI M, MIYAZAKI T, YOKOI N, TAKASHIMA H, WATANABE M, WATANABE O, FUKATA M. Autoantibodies to epilepsy-related LGI1 in limbic encephalitis neutralize LGI1-ADAM22 interaction and reduce synaptic AMPA receptors. J Neurosci. 2013;33(46):18161-74.
- OYANGUREN B, SANCHEZ V, GONZALEZ FJ, DE FELIPE A, ESTEBAN L, LOPEZ-SENDON JL, GARCIA-BARRAGAN N, MARTINEZ-SAN MILLAN J, MASJUAN J, CORRAL I. Limbic encephalitis: a clinical-radiological comparison between herpetic and autoimmune etiologies. Eur J Neurol. 2013;20(12):1566-70.
- PETIT-PEDROL M, ARMANGUE T, PENG X, BATALLER L, CELLUCCI T, DAVIS R, MCCRACKEN L, MARTINEZ-HERNANDEZ E, MASON WP, KRUER MC, RITACCO DG, GRISOLD W, MEANEY BF, ALCALA C, SILLEVIS-SMITT P, TITULAER MJ, BALICE-GORDON R, GRAUS F, DALMAU J. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol. 2014;13(3):276-86.
- POLLAK TA, BECK K, IRANI SR, HOWES OD, DAVID AS, MCGUIRE PK. Autoantibodies to central nervous system neuronal surface antigens: psychiatric symptoms and psychopharmacological implications. Psychopharmacology (Berl). 2016;233(9):1605-21.
- RASCHILAS F, WOLFF M, DELATOUR F, CHAFFAUT C, DE BROUCKER T, CHEVRET S, LEBON P, CANTON P, ROZENBERG F. Outcome of and prognostic factors for herpes simplex encephalitis in adult patients: results of a multicenter study. Clin Infect Dis. 2002;35(3):254-60.
- SANSING LH, TUZUN E, KO MW, BACCON J, LYNCH DR, DALMAU J. A patient with encephalitis associated with NMDA receptor antibodies. Nat Clin Pract Neurol. 2007;3(5):291-6.
- SCHMITT SE, PARGEON K, FRECHETTE ES, HIRSCH LJ, DALMAU J, FRIEDMAN D. Extreme delta brush: a unique EEG pattern in adults with anti-NMDA receptor encephalitis. Neurology. 2012;79(11):1094-100.
- SWEENEY M, GALLI J, MCNALLY S, TEBO A, HAVEN T, THULIN P, CLARDY SL. Delayed LGI1 seropositivity in voltage-gated potassium channel (VGKC)-complex antibody limbic encephalitis. BMJ Case Rep. 2017;2017.
- THIEBEN MJ, LENNON VA, BOEVE BF, AKSAMIT AJ, KEEGAN M, VERNINO S. Potentially reversible autoimmune limbic encephalitis with neuronal potassium channel antibody. Neurology. 2004;62(7):1177-82.
- TITULAER MJ, HOFTBERGER R, IIZUKA T, LEYPOLDT F, MCCRACKEN L, CELLUCCI T, BENSON LA, SHU H, IRIOKA T, HIRANO M, SINGH G, COBO CALVO A, KAIDA K, MORALES PS, WIRTZ PW, YAMAMOTO T, REINDL M, ROSENFELD MR, GRAUS F, SAIZ A, DALMAU J. Overlapping demyelinating syndromes and anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol. 2014;75(3):411-28.
- TITULAER MJ, MCCRACKEN L, GABILONDO I, ARMANGUE T, GLASER C, IIZUKA T, HONIG LS, BENSELER SM, KAWACHI I, MARTINEZ-HERNANDEZ E, AGUILAR E, GRESA-ARRIBAS N, RYAN-FLORANCE N, TORRENTS A, SAIZ A, ROSENFELD MR, BALICE-GORDON R, GRAUS F, DALMAU J. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157-65.
- TITULAER MJ, SOFFIETTI R, DALMAU J, GILHUS NE, GIOMETTO B, GRAUS F, GRISOLD W, HONNORAT J, SILLEVIS SMITT PA, TANASESCU R, VEDELER CA, VOLTZ R, VERSCHUUREN JJ, EUROPEAN FEDERATION OF NEUROLOGICAL S. Screening for tumours in paraneoplastic syndromes: report of an EFNS task force. Eur J Neurol. 2011;18(1):19-e3.
- TSUTSUI K, KANBAYASHI T, TAKAKI M, OMORI Y, IMAI Y, NISHINO S, TANAKA K, SHIMIZU T. N-Methyl-D-aspartate receptor antibody could be a cause of catatonic symptoms in psychiatric patients: case reports and methods for detection. Neuropsychiatr Dis Treat. 2017;13:339-345.
- VAN SONDEREN A, ARINO H, PETIT-PEDROL M, LEYPOLDT F, KORTVELYESSY P, WANDINGER KP, LANCASTER E, WIRTZ PW, SCHREURS MW, SILLEVIS SMITT PA, GRAUS F, DALMAU J, TITULAER MJ. The clinical spectrum of Caspr2 antibody-associated disease. Neurology. 2016;87(5):521-8.
- VENKATESAN A, TUNKEL AR, BLOCH KC, LAURING AS, SEJVAR J, BITNUN A, STAHL JP, MAILLES A, DREBOT M, RUPPRECHT CE, YODER J, COPE JR, WILSON MR, WHITLEY RJ, SULLIVAN J, GRANEROD J, JONES C, EASTWOOD K, WARD KN, DURRHEIM DN, SOLBRIG MV, GUO-DONG L, GLASER CA, INTERNATIONAL ENCEPHALITIS C. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013;57(8):1114-28.
- VIACCOZ A, DESESTRET V, DUCRAY F, PICARD G, CAVILLON G, ROGEMOND V, ANTOINE JC, DELATTRE JY, HONNORAT J. Clinical specificities of adult male patients with NMDA receptor antibodies encephalitis. Neurology. 2014;82(7):556-63.
- VINCENT A, BUCKLEY C, SCHOTT JM, BAKER I, DEWAR BK, DETERT N, CLOVER L, PARKINSON A, BIEN CG, OMER S, LANG B, ROSSOR MN, PALACE J. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain. 2004;127(Pt 3):701-12.
- VINCENT A, IRANI SR. Caspr2 antibodies in patients with thymomas. J Thorac Oncol. 2010;5(10 Suppl 4):S277-80.
- VITALIANI R, MASON W, ANCES B, ZWERDLING T, JIANG Z, DALMAU J. Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann Neurol. 2005;58(4):594-604.
- VORA NM, HOLMAN RC, MEHAL JM, STEINER CA, BLANTON J, SEJVAR J. Burden of encephalitis-associated hospitalizations in the United States, 1998-2010. Neurology. 2014;82(5):443-51.
- WEIL AA, GLASER CA, AMAD Z, FORGHANI B. Patients with suspected herpes simplex encephalitis: rethinking an initial negative polymerase chain reaction result. Clin Infect Dis. 2002;34(8):1154-7.
- WIESER S, KELEMEN A, BARSI P, VINCENT A, BORBELY C, RASONYI G, MUELLER S, HESS K, WIESER HG, HALASZ P. Pilomotor seizures and status in non-paraneoplastic limbic encephalitis. Epileptic Disord. 2005;7(3):205-11.
|
|