
|
|
|
CASE REPORT / CAS CLINIQUE
A CLINICO-PATHOLOGICAL EVALUATION OF TWO PATIENTS PRESENTING WITH THE NEUROMYELITIS OPTICA SYNDROME.
UNE ÉVALUATION CLINICOPATHOLOGIQUE DE DEUX PATIENTS PRÉSENTANT AVEC LE SYNDROME NEUROMYELITIS OPTICA.
E-Mail Contact - BHIGJEE Ahmed Iqbal :
BHIGJEE@ukzn.ac.za
ABSTRACT The discovery of the Aquaporin 4 (AQP4) antibody in patients with neuromyelitis optica (NMO) has expanded clinical spectrum of disorders associated with this antibody. It has also become clear that NMO can be a paraneoplastic manifestation of an underlying malignancy. We report on the pathological changes in the spinal cord in an NMO patient who underwent a biopsy for a suspected spinal tumour. We also present the findings in the testicular tumour of a patient who initially presented with NMO. In the first patient we demonstrate the loss of AQP4 staining and in the latter case we demonstrate the expression of AQP4 by tumour cells. Key words: Aquaporin 4 antibodies, NMO, paraneoplastic disorder INTRODUCTION Neuromyelitis Optica (NMO) or Devic’s disease is a central nervous system inflammatory disorder characterised by bilateral optic neuritis and a longitudinally extensive transverse myelitis. The optic neuritis may occur in each eye simultaneously or sequentially, and the myelitis may precede or succeed the optic neuritis. A major step forward in the understanding of NMO and its distinction as a separate entity from MS was the discovery of antibodies against the aquaporin-4 (AQP-4) water channel in the serum of many patients with NMO (4,5). Following this discovery it became apparent that the phenotypic presentations of NMO was much wider and included unilateral optic neuritis, recurrent optic neuritis, relapsing myelitis and asymptomatic brain lesions. Appreciation of non optico-spinal presentations in antibody positive patients, such as the area postrema, brainstem and diencephalic syndromes led to further refinement of the 2006 diagnostic criteria and the use of the term neuromyelitis optica spectrum disorders (NMOSD). An international panel has produced consensus diagnostic criteria for NMOSD (15). We describe the clinical and detailed pathological findings in two patients with NMO. The one patient was thought to have spinal tumour and underwent a spinal cord biopsy. The second patient presented with NMO and was later found to have a testicular tumour. PATIENTS Case 1 A 19 year old HIV negative woman was referred from a peripheral hospital where she presented five days earlier with weakness and pain in all her limbs which progressed over 24 hours. She also noticed difficulty in passing urine but had no visual disturbance or any other neurological complaint. There was no past history of note. On examination she was afebrile with no rash or lymphadenopathy. The mental state and cranial nerves were normal. The tone was decreased in the upper limbs but increased in the lower limbs. There was asymmetrical weakness of all limbs, more distally in the upper limbs and more proximally in the lower limbs. The upper limb tendon jerks were absent. The knee jerks were brisk and the ankle jerks were normal. Pinprick was impaired in all limbs without a clear sensory level. Joint position sense was impaired in all limbs. Vibration sense was absent in the legs. An MRI of the spine revealed a heterogeneous intramedullary cervical cord hyperintense lesion extending from the obex up to T3 level. There was associated cord expansion. Patchy intramedullary enhancement is present in this region (figures 1A-C). The rest of the spinal cord was normal. The radiological diagnosis was that of a cervical cord astrocytoma. Routine blood tests were unhelpful. A blood sample was taken for the AQP-4 antibody test. At surgery no clear tumour was identified but biopsies of suspicious areas were taken. About a month later, the AQP-4 antibody test was resulted as positive. Case 2 A 27 year old HIV negative man presented in December 2013 with acute onset headache and vomiting. Ten days later he developed urinary retention and progressive weakness of the legs. About three weeks later he noticed progressive loss of vision in both eyes. Examination revealed no perception to light in the right eye and counting fingers in the left eye. The margins of both optic discs were blurred. Upper limb power was normal. He had a grade 3-4/5 paraparesis. MRI of the brain showed hyperintensities in the right brachium pontis and right cerebellum. Both optic nerves were thickened with enhancement. The MRI of the spine showed irregular hyperintensities in the cervico-thoracic cord. The CSF was acellular and oligoclonal bands were absent. The anti AQP4 antibody test was negative. He was given 5 days of intravenous methylprednisone followed by oral prednisone and azathioprine 100mg daily. At the August 2014 follow up the power in the legs was normal and his vision improved to 6/9. In May 2015 he presented with a painless right testicular mass. Histology confirmed a non-seminomatous germ cell tumour with embryonal and yolk sac components. He underwent chemotherapy. The anti Ma2/Ta antibody done much later when there was no evidence of tumour was weakly positive. PATHOLOGICAL STUDIES Materials and Methods The specimens for histopathological evaluation were fixed in 10% formalin, sectioned and processed overnight for 8 hours in a VIP6 automated processor using a xylene-free protocol. Following paraffin wax-embedding, tissue blocks were sectioned at 4um and stained with haematoxylin and eosin using standard protocols for histopathological appraisal. Sections of the spinal cord biopsy were also stained with luxol fast blue and Sevier Munger histochemical stains. Aquaporin 4 (Sigma; polyclonal, 1:250 dilution), CD30 (Roche, Clone BerH2, Dilution: 1:300) and alpha-foetoprotein (Sigma; Clone C3; Dilution 1:300) immunohistochemistry was undertaken on 2um formalin fixed paraffin-embedded sections on the Ventana Benchmark Ultra automated platform. While CD30 and alphafoetoprotein immunostaining required heat-assisted microwave antigen retrieval, per protocol, antigen retrieval was not performed for AQP4, but overnight antibody incubation at 4°Celsius was undertaken. Diaminobenzidine served as the chromogen for antibody visualisation for all immunostains. The positive control tissues included renal tissue as a general control and age, sex and site-matched cervical spinal cord tissue as site-specific control. The primary antibody was omitted in negative controls. RESULTS Patient 1: Spinal cord biopsy. Sections of the lesional spinal cord tissue fragments demonstrated prominent demyelination, vacuolar change, scattered foamy histiocytes, neutrophils, lymphocytes, eosinophils and plasma cells (Figures 2A, B). Small thick-walled vessels were present (figure 2A). The lymphocytes were dominantly of a CD3 immunophenotype and a mixed CD4/CD8 immunophenotype. Granulomas or necrotic foci were not evident. Luxol fast blue and Sevier Munger stains confirmed demyelination and axonal loss (figures 2C & D). There was an absence of AQP4 and glial fibrillary acidic protein staining in the demyelinated foci. Increased vessel wall AQP4 staining was noted (figure 2E). Control cervical spinal cord sections demonstrated diffuse cytoplasmic AQP4 staining in the grey and white matter (Figure 2F). The pathological features supported a diagnosis of NMO. Patient 2: Testicular tumour. The testicular tumour weighed 890g and measured 11x8x7cm. There were large areas of tumour necrosis and haemorrhage. Microscopically the tumour demonstrated a mixed germ cell tumour composed of yolk sac tumour (60%) (Figures3A-C) and embryonal carcinoma (40%) (Figures 3D-F). AQP4, identified in both components, had a dominant subapical linear in addition to cytoplasmic distribution in the embryonal carcinoma component (Figure 3F) and a granular cytoplasmic immunoprofile in the yolk sac component (Figure 3C). A similar AQP4 immunoprofile was noted in intra-lymphatic tumour. DISCUSSION Spinal cord Pathology The histopathological spectrum of NMO encompasses acute and chronic injury patterns (1,6). Two distinctive acute histomorphological profiles of NMO are described in the spinal cord .5 The classic parenchymal changes include perivascular demyelination, axonal destruction, conspicuous astrocytic and identifiable oligodendrocytic loss, and grey and white matter necrosis, the inflammatory component is composed of myelin-rich macrophages and a variable perivascular infiltrate neutrophils, eosinophils, and T and B-lymphocytes (6,7,14). In the second form of acute NMO, myelin vacuolation in the absence of frank demyelination, limited axonal injury, variable astrocytic and microglial activity and granulocytic infiltration are seen (6,7,14). Chronic lesions are typified by variable spinal cord atrophy, gliosis and cystic change. Remyelination and fibrotic vascular mural thickening may also be noted, the former evidence by the presence of thinly myelinated axons. Autopsy profiling of spinal cord lesions facilitate the identification of the full house’ features of NMO, but ante-mortem biopsies are limited by biopsy size and representivity, as in the present study (Patient 1). In one fragment vacuolar change, demyelination and macrophages are noted at one edge and a fibro-inflammatory response with thick-walled vessels is noted in both. The inflammatory response included scattered granulocytes a mixed but sparse B and T lymphocytic response. Plasma cells were the most sparse inflammatory cell type. Given the mixed demyelinating and variable inflammatory response and the small biopsy volume, NMO staging is not possible. However, the loss of astrocytes and AQP4 in the early and late lesions is supportive of a pathogenetic role of astrocyte and AQP4 targeting in NMO (6), HIV myelopathy is characterised by vacuolar change and inflammatory infiltration (15). In HIV-infected patients with transverse myelitis, AQP4 testing is mandatory for confirmation of the underlying aetiopathogenesis of the vacuolar-inflammatory alterations. NMO as a Paraneoplastic Presentation Following the discovery of the anti AQP4 antibody, Pittock et al identified a thymic carcinoma in an NMO patient (12). A subsequent review by the Mayo Group found an association between NMO and a variety of other malignancies including breast, bronchus, thyroid, carcinoid and lymphoma (11). Our patient (case 2) presented with NMO first and became aware of a testicular mass about 17 months later. The anti AQP4 antibody test done at the time of his neurological presentation was negative. The neurological deficits improved on steroids and azathioprine. He underwent orchidectomy and chemo therapy and currently has no evidence of tumour. Anti Ma2 antibodies has been described in patients with typical limbic encephalitis but it has become apparent that the neurological presentation can be more varied. Other presentations include excessive daytime sleepiness, vertical ophthalmoparesis and cerebellar dysfunction. To the best of our knowledge an NMO presentation has not been described. The role of aquaporins in tumour behaviour and patient outcomes is multifaceted. Strongly expressed in a wide spectrum, especially of aggressive, tumour types, aquaporins are new players at local tumour sites in cell proliferation and migration tumour angiogenesis and oedema and as a potential underpinning cause of paraneoplastic syndromes (10), including NMO. In the craniospinal axis, aquaporins are upregulated in astrocytomas including glioblastomas, oedema-producing meningiomas and subependymomas (3,8,9). AQP4 has a direct invasive role in cancers (10, 13), including lung , biliary tract and gastric adenocarcinomas. In addition, bronchogenic and mammary adenocarcinomas, neuroendocrine carcinomas , thymic carcinoma , lymphoma , leukaemia and ovarian teratomas are implicated in AQP4-induced paraneoplastic syndromes (2). In germ cell tumours, AQP4 has, to date, been reported exclusively in the neural component of a teratoma (2). CONCLUSION This report adds to the description of the spinal cord pathology in NMO and further confirms that NMO can be a paraneoplastic presentation. 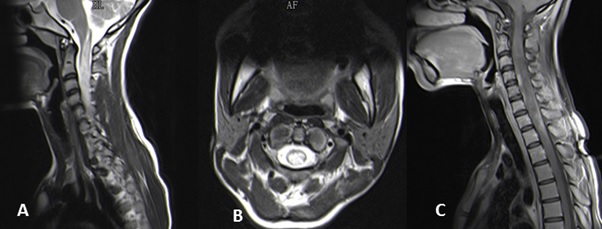 Figure 1 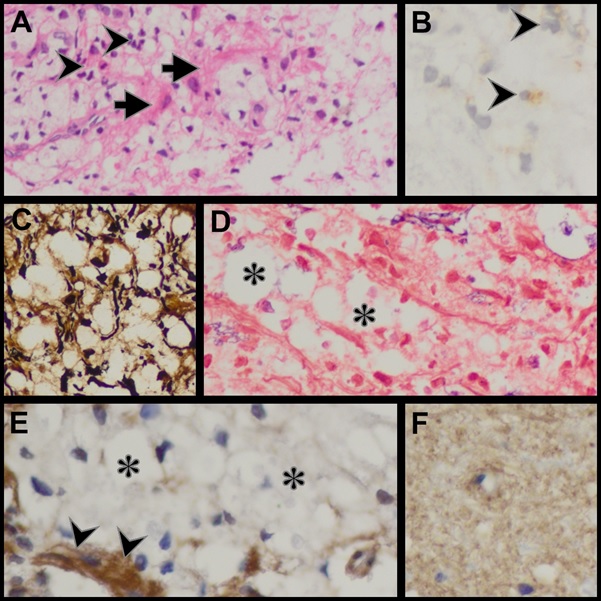 Figure 2
REFERENCES
|
© 2002-2018 African Journal of Neurological Sciences.
All rights reserved. Terms of use.
Tous droits réservés. Termes d'Utilisation.
ISSN: 1992-2647
