|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
CASE REPORT / CAS CLINIQUE
AUTONOMIC DYSREFLEXIA WITHIN 24 HOURS OF SPINAL CORD INJURY
DYSREFLEXIE AUTONOME DANS LES 24 HEURES SUIVANT UN TRAUMATISME MEDULLAIRE
E-Mail Contact - HASHEELA Toivo :
tukunah@yahoo.com
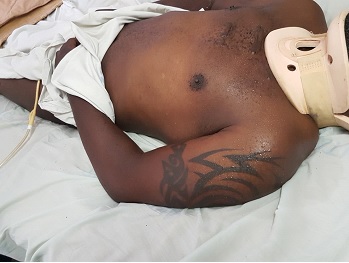
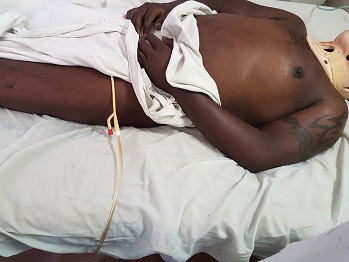
ABSTRACT Background: Autonomic dysreflexia (AD) is a potentially life-threatening condition, characterized by episodes of dangerously elevated systolic blood pressures of up to 300 mmHg that typically affects quadriplegic and high paraplegic patients with spinal cord lesions above the T6 segment. Such lesions lie above the origin of the splanchnic sympathetic innervation and therefore interrupt their supraspinal regulation. Case report: The condition usually occurs in the chronic stages of spinal cord injury, it rarely occurs in the acute phase post-injury. This case report describes a patient who developed AD within 24 hours of injury. Conclusion: To the best of our knowledge, no such case has been reported before in literature. KEYWORDS: Autonomic dysreflexia, exaggerated autonomic responses, splanchnic innervation. INTRODUCTION Autonomic dysreflexia (AD) is a potentially life-threatening medical emergency. It affects patients with spinal cord lesions above the origin of the major splanchnic outflow, T6 to L2, although it has also been reported with lesions as low as T10 (9). Although often underdiagnosed, it has been reported to affect up to 90% of patients with high thoracic and cervical injuries in some series (2). It manifests in the form of exaggerated sympathetic responses below the level of injury, and predominance of parasympathetic activity above. The classical triad of symptoms seen in up to 85% at presentation comprises of (4): Acute elevation of arterial BP, at least >20 mmHg above baseline, pounding headache, hyperhidrosis and flushing in dermatomes above lesion, accompanied by pilo-erection, skin pallor and dryness in dermatomes below. Other signs and symptoms may include: cardiac arrhythmias, anxiety, nasal congestion, mydriasis and blurred vision (4). The underlying pathology is loss of supraspinal control over sympathetic preganglionic neurons that lie caudal to the lesion. As a result, there is loss of ability to vasodilate the splanchnic vascular bed by central command when needed. Additionally, these sympathetic preganglionic neurons become hyper-responsive to stimulation. Stimulus may arise from otherwise non-noxious or only mildly noxious stimuli, such as an overdistended bladder due to a kinked/blocked urinary catheter (most common cause) (73%), bladder/kidney stones and urinary tract infections (3%), faecal impaction (12%), decubitus ulcers (4%), deep vein thrombosis, ingrown toenail, fractures, menstruation, haemorrhoids, or invasive procedures (cystoscopy, debridement, suprapubic tube insertion) (1,6,7). Even seemingly benign stimuli such as tight clothing or shoelace have been reported to trigger AD. Formation of aberrant synaptic connections and intraspinal sprouting of primary afferent nociceptive fibers during axonal regeneration of the severed axons may contribute to the hyper-responsiveness (3). Additionally, altered sensitivity of peripheral alpha-adrenergic receptors to catecholamines has also been implicated as a possible contributor in the mechanism of development of AD (8). Ever since the initial landmark description of AD by Hilton in 1860 (8), the condition was believed to occur exclusively during the chronic stages of spinal cord injury. According to more recent literature, this condition rarely occurs within the first 12–16 weeks post-injury (4). The earliest first episode described so far was reported on the 4th day post-injury (8). This case report gives an account of a patient who presented with signs and symptoms consistent with features of AD within 24 hours of injury. CASE PRESENTATION A 42-years-old, previously well, male was brought to the emergency department (ED) after a motor vehicle accident. His complaints were severe posterior neck pain, and weakness and paresthesia of both upper limbs. Although the patient was conscious with a Glasgow Coma Score of 15/15, the presence of neurologic deficits, posterior midline cervical tenderness and a history of alcohol intoxication precluded the possibility of reliably clearing his cervical spine on the basis of history and clinical examination alone. Therefore, as per NEXUS (National Emergency X-Radiography Utilization Study) protocol, imaging in the form of cervical spine x-rays including antero-posterior, lateral and open mouth views were requested. The lateral view revealed anterior subluxation of C5/6, with grade I spondylolisthesis according to the Meyerding classification (Figure 1). A detailed neurological examination further revealed that the patient had posterior midline cervical tenderness on palpation. Hypertonia and hypereflexia were noted in the lower limbs. Sensation was spared in all dermatomes as evidenced by sacral sparing. No priapism was noted to suggest complete spinal cord injury. The patient was deemed not in spinal shock as the bulbocarvenosus reflex was present. A final diagnosis of incomplete cervical spinal cord injury ASIA D, according to the American Spinal Injury Association (ASIA) impairment scale, with central cord syndrome, following traumatic C5/6 subluxation was made. It was further noted that, contrary to the usual finding were patients with spinal cord injuries tend to have low blood pressures (i.e. below 120/90) due to neurogenic shock, the blood pressure readings recorded by the paramedics during the pre-hospital phase and during ambulance transfer demonstrated an exponentially upward trend, i.e. 125/90 mmHg, 138/90 mmHg, and 158/99 mmHg; respectively. Upon arrival in the ED, it was further noted that the patient had developed severely elevated blood pressures of up to 241/147 mmHg. While still awaiting a physician’s review in the ED for the management of the hypertensive crisis, it was noted that the blood pressures began to gradually decline immediately after bladder catheterization and transfer off the spine immobilization board were done, (Point (a), Figure 2). Although the patient was eventually started on anti-hypertensive medications, those did not effectively control the hypertension. Definitive management included immediate closed reduction with Gardner-Wells tongs followed by a planned single-level Anterior Cervical Discectomy and Fusion (ACDF) at C5/6. Unfortunately, regardless of all efforts, the patient deceased on the 28th day post-admission. DISCUSSION Most authors characterize an episode of AD as an increase in systolic blood pressure of at least 20% (8). Consistent with this definition, our patient’s systolic blood pressures demonstrated a sudden increase of at least 72%, from an average baseline SBP of 140 mmHg during ambulance transfer to 241 mmHg upon arrival in the ED. Furthermore, the patient’s blood pressures began to decline gradually immediately after bladder catheterization and evacuation of 370 ml of dilute urine. Bladder distension has been described as the most common precipitating stimulus for AD (4). A full bladder in this patient is an expected finding given the history that patient was on his way from a night out where alcohol, a potent diuretic, had been consumed. Also notably, the point in time at which the blood pressures began to decline (point (a) on the line graph in figure 2) coincides with the point at which the patient was transferred off the spine immobilization board, further suggesting possible elimination of a potential precipitating stimulus, i.e. pain from skin pressure points. Differential diagnosis for the BP elevations and kidney failure in this patient include pre-existing essential hypertension with target organ damage, however, the patient’s past medical history did not reveal pre-existing hypertension. Furthermore, the diagnosis of AD in this patient was further strongly suggested by the following observations: hyperhidrosis limited to dermatomes above the level of injury (Figures 3a and 3b) ; Reproducible resolution of episodes hypertensive crises after removal of TED (thrombo-embolus deterrent) stockings that had been refractory to anti-hypertensive medication (points (d) and (f) on line graph in figure 2). (NB: Tight clothing is a known precipitating factor). The differential diagnosis for the acute kidney injury (AKI) observed on day 4 post-injury, (Figure 4), included acute tubular necrosis subsequent to rhabdomyolysis and myoglobinemia from extensive blunt soft tissue injuries. However, urinalysis did not show myoglobinuria of corresponding magnitude and pattern. Thus, although the latter could have contributed, this acute kidney injury was deemed more likely a result of end organ damage by the recurrent episodes of severely elevated blood pressures. Furthermore, it was noted that the AKI did not respond adequately to several sessions of dialysis. This further suggests that the inciting pathology was more likely to be an on-going process rather than a discrete event like soft tissue trauma. CONCLUSION Contrary to the traditional perception that occurrence of AD is limited to the chronic stages of SCI, not only can the condition occur in the acute phase of SCI as well, but the first episode of AD can occur within 24 hours, immediately after injury.
Table: Daily Urea and electrolytes results
REFERENCES
|
© 2002-2018 African Journal of Neurological Sciences.
All rights reserved. Terms of use.
Tous droits réservés. Termes d'Utilisation.
ISSN: 1992-2647