
|
|
|
TECHNIQUES
LAPAROSCOPIC INSERTION OF LUMBAR PERITONEAL SHUNTS FOR IDIOPATHIC INTRACRANIAL HYPERTENSION
INSERTION LAPAROSCOPIQUE DES SHUNTS LOMBAIRES PERITONEAUX POUR L'HYPERTENSION INTRACRANIENNE IDIOPATHIQUE
E-Mail Contact - NAIDOO Dinesh :
dineshnaidoo@yahoo.com
ABSTRACT Background Objective Conclusion INTRODUCTION Idiopathic intracranial hypertension [IIH] (also called benign intracranial hypertension or pseudotumor cerebri) is a condition characterised by raised intracranial pressure in the absence of a structural or vascular lesion, or ventriculomegaly or other identifiable causes of raised intracranial pressure such as certain medications.Idiopatic intracranial hypertension is usually diagnosed by the finding of elevated opening pressures when performing a lumbar puncture. The condition is however far from benign and causes blindness in up to 10% of affected patients, indeed most affected people have some degree of visual loss (3), which may be uncovered only with formal visual perimetry testing. The (modified Dandy) criteria for the diagnosis of IIH include :symptoms of increased intracranial pressure, no localising neurological sign or a false localising sign such as an abducens nerve palsy, an awake and alert patient, normal imaging studies without evidence of dural sinus thrombosis, an elevated CSF pressure of more than 20-25 mmHg measured by performing a lumbar puncture with the patient in the lateral decubitus position, normal cerebrospinal fluid analysis and no other cause of elevated cerebrospinal fluid pressure (5). Some controversy exists with regard to the upper limit of CSF pressure with a belief that body weight influences cerebrospinal fluid pressure levels. There is, however, no convincing evidence for this belief (3, 14). Women are most affected by IIH. The patients are invariably obese and have several centimetres of fat between the skin and rectus abdominis fascia. (5, 6). Use of electrocautery through this thick layer of fat may lead to poor wound healing and seroma formation (6).Indeed obesity has been implicated in the pathogenesis of IIH. Both weight loss and bariatric surgery have been demonstrated to lower CSF opening pressure (5). IIH may be treated by observation only, by weight loss or with the use of carbonic anhydrase inhibitors or other diuretics (14). The condition is sometimes cured with repeated lumbar punctures. Surgery is considered when medical treatment fails, particularly with the worsening of visual symptoms or signs. Surgical options include cerebrospinal fluid (CSF) diversion and optic nerve fenestration. It is controversial whether either option is superior, and the choice of operation may depend on local expertise and resources (14). Cerebrospinal fluid diversion has the advantage of treating both headaches due to raised intracranial pressure and visual loss with a single procedure. Cerebrospinal fluid shunting may be accomplished by lumbarperitoneal or ventriculoperitoneal shunting. Lumbarperitoneal shunts are generally preferred as patients with idiopathic intracranial hypertension frequently have small “slit-like”, ventricles which make ventricular cannulation difficult. Further lumbarperitoneal shunts avoid the risks associated with intracranial surgery. Bariatric surgery has also been advocated as a cure for intracranial hypertension with higher durability than CSF-peritoneal diversion. Bariatric surgery has the additional benefit of ameliorating the other co-morbidities associated with obesity (14, 10).Bariatric surgery, however, will not be useful in patients with acutely deteriorating vision (16). Lumbarperitoneal shunts are traditionally placed through an open approach with the patient in the lateral decubitus position (6).The shunt is introduced into the lumbar subarachnoid space with a Touhy needle. The shunt is then tunnelled subcutaneously to the flank, and brought out into the open using a special tunnelling instrument. The distal end of the shunt is then introduced into the peritoneal cavity via a mini-laparotomy, using the same flank incision or, less commonly, by direct puncture with a trocar (16). CASE REPORT A 44 year old female patient with a chronic history of severe headaches and raised opening lumbar pressures was referred to the senior author after she developed a CSF leak from her ear following a middle ear operation. The headaches responded briefly to lumbar CSF aspiration but would invariably return some time after the lumbar puncture. The patient weighed 106 kg and stood 1,59m tall .This equated to a body mass index (BMI) of 42. An external lumbar drain was inserted for 5 days with resolution of both the patient’s headaches and the CSF leak. Both the CSF leak and headaches however summarily returned on removal of the drain. A lumbarperitoneal shunt was inserted via a right flank mini- laparotomy. This procedure was accompanied by significant headache relief and cessation of the CSF leak. An incisional hernia developed approximately 9 months later. The hernia was mildly symptomatic and conservative treatment was advised. The patient sought a second opinion and underwent a herniorraphy at a different institution. The patient returned to the senior author twelve months after the diagnosis of the incisional hernia (and four months after its repair) complaining of a large fluid collection in the right. iliac fossa which clinically appeared to be extra-peritoneal. CT scan of the abdomen demonstrated the shunt to be in an extra-peritoneal location with a large extra-peritoneal fluid collection in the right iliac fossa and no hernia recurrence. The patient at this stage was afebrile with normal laboratory functions. The patient was returned to the operating room under general anaesthetic. The CSF pseudocyst was drained and the shunt was re-inserted into the peritoneal cavity via a right para-umbilical area mini-laparotomy. The pseudocyst fluid was clear and colourless and approximately 200 ml in volume. CSF obtained from the distal end of the shunt was unremarkable on laboratory analysis. Approximately 8 days later the patient developed a severe pyrexial illness with a temperature of 39ºC with wound and gross subcutaneous tissue sepsis. This necessitated removal of the lumbarperitoneal shunt in its entirety. The shunt material was submitted for analysis and grew a coagulase negative staphylococcus sensitive to multiple antibiotics. The CSF culture yielded no growth and no cellular response. Blood cultures were negative. The patient received intravenous antibiotics for 8 days with normalisation of her temperature during the first 24 hours post shunt removal. The patient was discharged home on acetazolamide which is a carbonic anhydrase inhibitor. A new lumbarperitoneal shunt was inserted laparoscopically some 3 months later; after the patient’s symptoms of IIH returned and a repeat lumbar puncture demonstrated an opening pressure of 35 cm water. The patient has suffered no complications approximately 18 months following laparoscopic shunt insertion with significant amelioration of her headaches and no recurrence of the CSF otorrhoea. SURGICAL/OPERATIVE PROCEDURE The shunt is performed under general anaesthesia. The patient is initially place in the left lateral position. A longitudinal or transverse incision down to the lumbar fascia is made over the L4/5 interspace. The lumbar catheter is introduced into the lumbar subarachnoid space via a Touhy needle. The distal end of the catheter is tunnelled to the right flank and brought out into the open through a small incision. The lumbar wound is closed definitively and the flank incision and exposed shunt are dressed in a sterile fashion. The patient is now placed in the supine position. A 10mm viewing port is introduced under direct vision in the immediate para-umbilical area. The lumbar catheter is introduced via a 5mm port placed in the right upper quadrant. The 5mm port is removed and the peritoneal catheter is then tunnelled subcutaneously to the right flank incision. The lumbar and peritoneal catheters are joined together with an intervening, usually flow controlled, valve. Any remaining slack in the now single lumbarperitoneal catheter is pulled into the peritoneal cavity via grasping forceps inserted through a 5mm port introduced into the left upper quadrant. The grasping forceps can also be used to place the shunt tip in an area free of adhesions and/or omentum. The shunt tip can also be held up to better visualise CSF drainage. All wounds are closed in a single layer using an interrupted non-absorbable suture  Figure 1 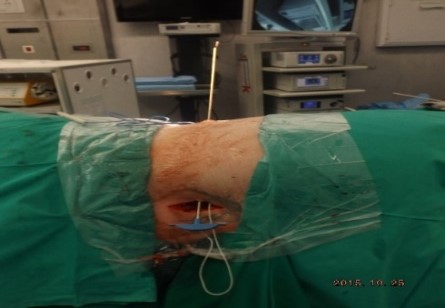 Figure 2  Figure 3  Figure 4  Figure 5 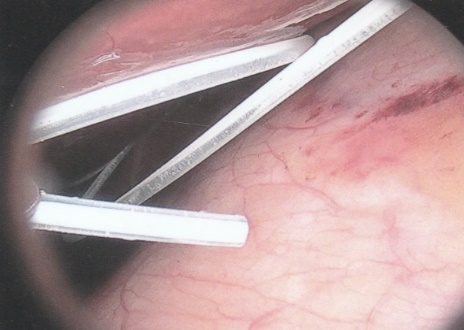 Figure 6 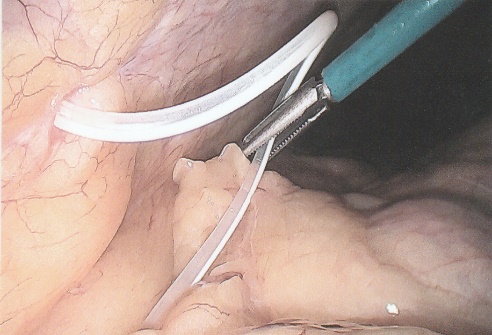 Figure 7 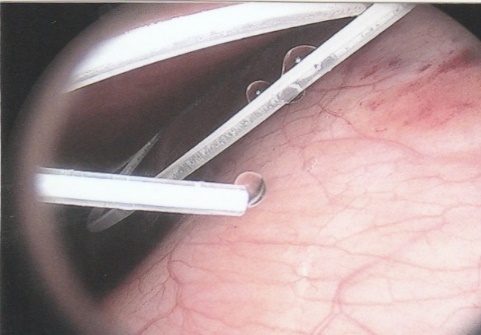 Figure 8 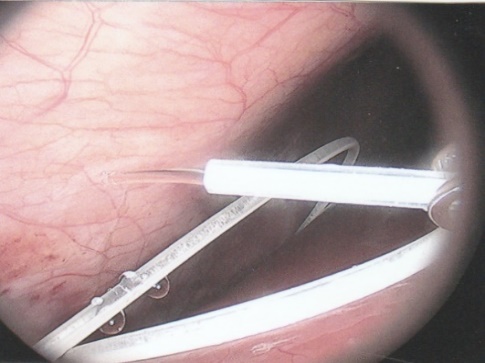 Figure 9 RESULTS Our series consists of seven laparoscopically inserted lumbarperitoneal shunts. Five female patients with IIH had failed medical management and were referred for CSF diversion. Only 1 of the 5 patients had significant visual morbidity in the form of severe papilledema and severely constricted visual fields. Four of the 5 female patients were morbidly obese and the 5th was obese. Two male patients underwent the procedure for normal pressure hydrocephalus after repeated lumbar punctures were shown to be associated with significant symptomatic improvement. Two complications occurred. In 1female patient the entire shunt migrated into the peritoneal cavity. This was the only valveless shunt placed. The shunt was retrieved laparoscopically and a new shunt with a flow regulated valve was inserted at the same sitting. A second patient presented with recurrent symptoms and an increased lumbar puncture opening pressure. A diagnostic laparoscopy was performed. This showed the shunt catheter to be deeply embedded in intraperitoneal fat with no CSF egress. The shunt was positioned in a different area of the peritoneal cavity with complete symptom resolution. DISCUSSION The commonest indication for the insertion of a lumbarperitoneal shunt is IIH (4). Other indications include communicating hydrocephalus, psuedomeningocoele, cerebrospinal fluid leak and normal pressure hydrocephalus. Patients with IIH are invariably severely obese. Indeed it is hypothesised that increased intra-abdominal pressure is pathophysiologically responsible for the development of idiopathic intracranial hypertension (10). Lumbarperitoneal shunts were first introduced in the 1950s .These initial shunts were associated with spinal arachnoiditis and scoliosis thought to be due to the polyethylene material used in the manufacture of the shunts. The incidence of arachnoiditis and scoliosis was drastically reduced with the introduction of silastic catheters in 1975 (4).Despite this improvement, lumbarperitoneal shunts have significant complications. Orthostatic hypotension can cause disabling headaches particularly when valveless shunts are used. The shunts may also migrate out of the abdomen or spine. In addition the shunt may become infected and/or obstructed, particularly at the peritoneal end. Lumbar shunts have been traditionally inserted into the peritoneum via a lateral minilaparotomy incision. We believe that inserting the peritoneal end of the catheter laparoscopically can decrease at least some of the complications associated with lumbarperitoneal shunting. The peritoneal end of the shunt can be placed under vision in an area free of adhesions or fat, theoretically reducing the incidence of early distal shunt obstruction. Patients with IIH are invariably obese, often morbidly so.Obesity is a risk factor for the development of an incisional hernia ( 1,2,8,12,13,15).Even though smaller “mini laparotomy” incisions are used for the traditional insertion of lumbarperitoneal shunts this may not necessarily reduce the incidence of incisional hernias (1 ).Incisional hernias occur at a rate of 11-23 % (1).Trocar site hernias occur at a rate of 0.2-3.1% (11 ).Although the incidence of trocar site herniation may be underestimated, trocar site hernias that result in aponeurotic defects of less than 10mm rarely result in bowel herniation or obstruction (9, 11). Shunt function can assessed by direct visualisation of CSF drainage. This can be done at the initial surgery or later in instances of suspected shunt obstruction. Such obstruction may also be remedied laparoscopically. Kato et al described a “complicated”, method of retroperitoneal shunt insertion, particularly for patients who have had previous abdominal surgery (17).However, in spite of our limited experience at this stage we did not find previous abdominal surgery to be a contraindication to laparoscopic shunt placement in the manner that we have described. Several groups have previously reported the use of laparoscopy for peritoneal catheter insertion beginning in 1983 (7).To our knowledge this is the first report of this technique in South Africa. Causha et al described their technique of laparoscopic shunt insertion using a single viewing port and a 10F introducer, although an additional port was required in some cases (7). Both ventriculoperitoneal and lumbarperitoneal shunts were inserted laparoscopically. This group also did not find previous abdominal surgery to be a contraindication to conventional laparoscopy. CONCLUSION We believe (on the basis of this small series) that the laparoscopic placement of lumbarperitoneal shunts is a safe and efficacious procedure with many advantages over the traditional minilaparotomy approach. The laparoscopic placement procedure should be particularly considered in obese and morbidly obese patients with IIH REFERENCES
|
© 2002-2018 African Journal of Neurological Sciences.
All rights reserved. Terms of use.
Tous droits réservés. Termes d'Utilisation.
ISSN: 1992-2647