
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
NEUROEPIDEMIOLOGY / NEUROEPIDEMIOLOGIE
NEUROEPIDEMIOLOGY OF KONZO A SPASTIC PARA-TETRAPARESIS OF ACUTE ONSET IN A NEW AREA OF THE DEMOCRATIC REPUBLIC OF CONGO
E-Mail Contact - TSHALA-KATUMBAY Désiré :
desire_tshala@yahoo.com
ABSTRACT Background Method Results Conclusion Mots clés : manioc, konzo, paraparésie spastique aigue, substances cyanogènes RESUME Description Objectif Methode Resultats Conclusion Keywords: acute spastic paraparesis, cassava, cyanogens exposure, konzo INTRODUCTION Spastic paraparesis has been documented both in local outbreaks and in endemic areas in different geographical areas of the world. Various factors have been associated with these epidemics or endemics; for instance, the retrovirus Human Tlymphotropic Virus, type I (HTLV-I) in HTLV-I-associated myelopathy (HAM)(1,47), the over-consumption, with a restrictive diet, of the grass-pea (Lathyrus Sativus) in lathyrism (5,12) and the consumption of insufficiently processed bitter cassava with a low intake of sulfur amino acids in konzo (11). A syndrome called tropical ataxic neuropathy (TAN) has also been associated with the consumption of insufficiently processed bitter cassava, though it has been clinically connected with predominantly sensory disturbances(10). In 1996, we made a neuroepidemiological study of an outbreak of a spastic paraparesis, suspect of konzo, in Bandundu province of the Democratic republic of Congo (DRC). The aim of the study was to determine if this outbreak was compatible with konzo, and to investigate if the disease was associated to the same possible causal factors as in previous studies in other parts of Africa. MATERIAL AND METHODS Study area Bandundu Province, in the Democratic Republic of Congo, is situated southeast of the capital Kinshasa (2°-8° south and 16°-20° east). It covers 295,000 km2 with a population of 3.7 million in 1984, according to national demographic statistics (6). Popokabaka Health Zone is located in the southwestern part of this province. It consists of a savanna tableland with poor sandy soils intersected by forested, relatively more fertile river valleys running in roughly south-north. The population lives mainly in villages, growing cassava as their major subsistence and cash crop. Due to a reported high prevalence of the disease at Masina Health Center, we selected the Masina catchment area (14 x 14 km), situated 90 km east of Popokabaka township as the study area (Fig.1). Methods The study was done in August 1996. With informed consent and assistance from village leaders, a demographic census was performed in all 11 villages in the area. The inhabitants were registered according to ethnic affiliation, sex, and age group (children <15 years, adults ³15 years). The population in each village was screened for konzo by examining all persons with walking difficulties identified by village leaders or health staff. The WHO criteria for konzo (15) were applied: a visible symmetric spastic abnormality when walking and/or running, a history of abrupt onset (< 1 week), a non-progressive course, in a formerly healthy person, showing bilaterally exaggerated knee and/or ankle jerks without signs of spinal disease. Those fulfilling the criteria for konzo were interviewed in Kiyaka, the local language, according to a standardized questionnaire regarding the time of onset and the diet at onset. Information on year of onset was cross-checked with dates on birth certificates available for neighboring children. Month of onset was determined by use of a local event calendar. Thereafter, konzo-affected persons were invited for a detailed neurological examination by a neurologist (D.T.) including evaluation of higher cerebral functions, as well as an evaluation of the cranial nerve function, and of motor, sensory, autonomic and cerebellar functions. The severity of the disease was graded according to the WHO classification (15) (mild form = walking without support, moderate form = using one or two sticks, severe form = unable to walk). Other clinical signs were recorded and the size of the thyroid gland assessed according to the new WHO classification (16): grade 0 = no goiter, grade 1 = palpable goiter, grade 2 = visible goiter. Konzo subjects who had died were traced and characterized through interviews with the nurse at the Health Center, relatives and neighbors. Focus group interviews (3) with 5-9 adult participants of mixed ages were performed in the nine largest villages. A set of open questions was introduced concerning the village, seasonal and annual variations in agriculture, cassava processing, cassava marketing, diet, and konzo, a well-known disease in the area. A new semi-quantitative field assay for urinary thiocyanate was developed and used to screen 20-30 urine samples in each village, for thiocyanate content. This new method is a modification of the previous thiocyanate method (8) suitable for field surveys and yielding immediate semi-quantitative results. It distinguishes ordinary concentrations (below 100 µmol/L) from elevated levels (above 300 µmol/L). A blood specimen and spot urine samples were collected from examined konzo patients. Specimens were analyzed at the Department of Clinical Chemistry at Uppsala University Hospital in Sweden. Serum was analyzed for prealbumin, albumin, C-Reactive Protein, creatinine and thiocyanate (8). Urine was analyzed for linamarin (2), thiocyanate (8) and sulfate (9). Serum and urinary thiocyanate and urinary linamarin served as indicators of cyanogen exposure, whereas albumin, prealbumin, and sulfate served as protein status indicators. Virological tests for HIV 1-2 (Behring ELISA) and HTLV I-II antibodies (ELISA/Wellcome) were carried out at the Department of Microbiology, Uppsala University Hospital. RESULTS The Masina Health Center catchment area consists of 11 villages with, in august 1996 a total of 490 households and 2,723 inhabitants (551 men, 755 women, 714 boys <15 years and 703 girls <15 years), giving a mean of 5.5 persons per household. The number of inhabitants in each of the 11 villages is given in Fig.1. Yaka was the only ethnic group in the area. Occurrence of konzo Of 152 persons with walking difficulties, 55 fulfilled the criteria of konzo, thus a prevalence of 20 per thousand inhabitants in the study area. The remainder (97/152) had disabilities of various other origins: pain in the joints of lower limbs (92), psychosomatic disorder (2), possible disk hernia (1), myositis (1), and foot injury (1). A typical history of konzo was also obtained for another 27 named persons, 12 of whom had moved to neighboring villages outside the catchment area or to the capital, Kinshasa. Two of these were visited in their homes in Kinshasa for interview and examination. The other 15 were deceased. Of the 82 persons thus identified as being affected by konzo, 20 were boys below 15 years of age at the time of onset, 23 were girls, 7 men aged 15 years or more, and 32 women aged 15 years or more. The age and sex distributions are presented in Fig. 2. The annual and monthly distributions of the onset of konzo are shown in Fig. 3 and 4, respectively. Clinical findings Symptoms at onset of konzo
Table 1 shows the motor findings in relation to the severity of konzo. All subjects had decreased muscle power in the extensors of the lower limbs. Two of the seven subjects severely affected by konzo had tetraparesis. Tendon reflexes were exaggerated in the lower limbs in 42 patients (74%), while in the remainder the reflexes were difficult to elicit due to joint contractures or ankylosis. Ankle clonus was found in 40 cases (70%) and was similarly difficult to elicit in the remainder. Babinski’s sign was found in 34 subjects (60%) and no response in the remainder. All patients except 6 (11%) with joint contractures, had symmetric clinical signs in their limbs. Ten subjects (18%) lacked cutaneous abdominal reflexes. Eight subjects (14%) presented a bilateral palmomental reflex. Speech problems of dysarthric type were found in 14/57 patients (25%) with either the moderate or severe form of konzo. Thirteen subjects (23%) with either mild, moderate, or severe konzo reported impaired visual acuity at the onset of the disease. Rotatory nystagmus was observed in 3 subjects (5%) with onset at 2, 8 and 12 years of age, having a mild or moderate form of konzo. One 11-year-old subject showed mental and physical retardation and had spastic tetraplegia with bilateral palmomental reflex and Babinski’s sign. He had contracted the disease 4 years earlier, in its mild form, but had two abrupt aggravations, one 3 years after the onset and the other 6 months before the study. Subsequently and simultaneously he lost the ability to walk, to speak and to swallow. He was still unable to walk at the time of the study. No cerebellar dysfunction was found in the patients. Sensory disturbances (touch, pain, position, and vibration) or autonomic symptoms (such as disturbance of urethra – and/or anal sphincter and sexual functions) were not found or reported, except for paresthesia and the sensation of electrical discharges in the lower limbs at onset. Other clinical signs Distal amyotrophy in arms and/or legs was found in 4 subjects (7%), and lumbar kyphosis in 3 cases (5%). Thirty-six patients (63%) had goiter; of these 27 (47%) had grade 1 goiter and 9 (16%) grade 2. Various signs of malnutrition (discolored hair, skin desquamation, edema) were found in 7 cases (12%). The diet The focus groups revealed that people relied on bitter cassava as their staple diet. Shortcuts in the processing of cassava roots are common – e.g. usually only 2 nights of soaking. Especially since 1992, shortcuts in processing had become common as a result of the intensive trade in cassava to Kinshasa. All the konzo patients ate the traditional cassava-flour based dough every day as staple. Cassava leaves were the most commonly eaten supplementary food. Meat and fish were not yet eaten daily in the villages. Lathyrus Sativus was not known at all or seen in the area. Laboratory findings Urinary thiocyanate testing by the semi-quantitative method in 213 randomly selected villagers in the area showed that 160 (75%) of the urine samples contained thiocyanate more than 300 µmol/L. Of the 38 blood samples, all proved negative to all four tested retroviruses (HIV-1, HIV-2, HTLV-I and HTLV-II). Creactive Protein was normal in all except one who had a slight increase (21 mg/L). The mean concentration of albumin was 28 (±5) g/L, all except one below the reference value of 40-52 g/L; of prealbumin 0.2 (±0.05) g/L, 28 of 38 patients being below the reference value of 0.225 g/L. The mean (±SD) concentration of thiocyanate was 502 (±153) mmol/L. Serum creatinine was clearly elevated (>100 mmol/L) in 21 of the 38 subjects, the range being 42-280 mmol/L. DISCUSSION This outbreak shared many characteristics with those previously described in Bandundu. Age and sex distributions and the seasonal variation are similar to those in earlier reports (11). The interview findings also confirm that the population in this area is using short-processed bitter cassava and that this short-processing has become more common as a consequence of intensified trade in cassava to Kinshasa. The high concentrations of thiocyanate in the urine of the general population confirm that the consumption of short-processed cassava exposes the people to dietary cyanogens. The main clinical sign of konzo is a spastic paraparesis, as previously described in several studies(11). The disease has a sudden onset, starting mainly with trembling in the legs. The handicap remains irreversible and may be exacerbated by further attacks, leading to severe disability. The disease is sometimes associated with other symptoms related to cranial nerve involvement (visual impairment) and/or pseudobulbar signs (speech or swallowing difficulties). Most subjects show a symmetrical clinical picture, except when joint contractures or ankylosis are present. The proportion of neurological signs increases with the severity of konzo. This involves mainly the addition of abnormalities in the upper limbs (increased tendon reflexes and /or decreased muscle power), leading to tetraplegia in severe cases. In this respect konzo shares similarities with the spastic diplegia of cerebral palsy. The nystagmus found in 3 subjects (5%) with onset at 2, 8, and 12 years of age raises the question whether it should be considered a sign of konzo. This symptom might be of clinical importance, since it has also been described in 2 patients with konzo in the Central African Republic (14). The palmomental reflex has not been reported in previous studies. Although it might support brain dysfunction, it is known that it can occur in healthy subjects in the general population. The mental retardation, found in one patient, raises the question whether konzo also affects mental capacity. Indeed, this subject lost his mental faculties as soon as he experienced two further attacks of konzo, which exacerbated his motor disability. On the other hand, this patient might have had an initial encephalopathy, and konzo could have impaired his condition further, or vice versa. Other clinical signs such as amyotrophy and lumbar kyphosis are associated with konzo. A central motor neurone dysfunction can lead to amyotrophy, whereas the typical toe-walk with knee flexion of konzo subjects can result in kyphosis as a compensatory phenomenon. The high proportion of goiter (63%) raises the question whether clinical signs of Iodine Deficiency Disorders (IDD) might be associated with konzo and make unclear, to some extent, the pathogenesis of certain symptoms, for example in case of mental retardation or nystagmus. The increased serum creatinine level has not been reported earlier and could be related to the fact that water was very scarce in the study area. Consequently, the low hydration of subjects might explain the high values observed, but this needs to be further evaluated. Laboratory findings demonstrated high cyanogens exposure in connection with low protein intake and the absence of retroviruses antibodies, as found in previous konzo study areas (11,13). Cyanogenic glucosides and their metabolites are therefore once again linked to konzo.
Table 1 : Motor findings in relation to severity of konzo
Table 2 : A subset of neurological symptoms found in relation to the severity of konzo
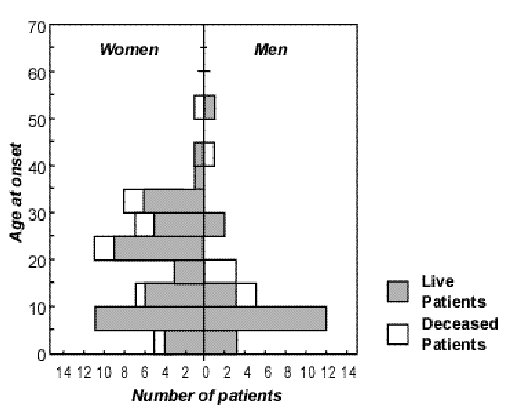 Figure 2
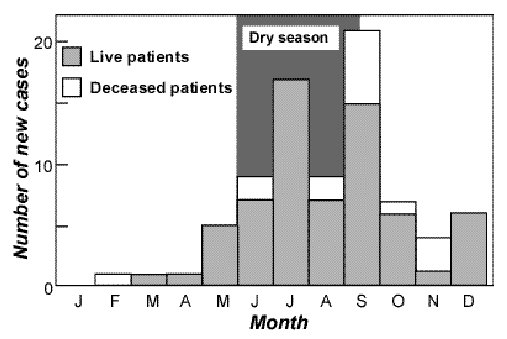 Figure 4 REFERENCES
|
© 2002-2018 African Journal of Neurological Sciences.
All rights reserved. Terms of use.
Tous droits réservés. Termes d'Utilisation.
ISSN: 1992-2647

