CLINICAL STUDIES / ETUDES CLINIQUES
NEUROLOGICAL SEQUELAE IN CHILDREN WITH PYOGENIC MENINGITIS IN A TERTIARY CENTRE IN LAGOS (NIGERIA)
SEQUELLES NEUROLOGIQUES CHEZ DES ENFANTS ATTEINTS D'UNE MENINGITE PURULENTE DANS UN CENTRE TERTIAIRE A LAGOS (NIGERIA)
- Department of Paediatrics, Lagos University Teaching Hospital P. M. B. 12003 Lagos, Nigeria
ABSTRACT
Background
Neurological sequelae following childhood bacterial meningitis are common particularly in the presence of delayed diagnosis and treatment. The latter is commonplace with meningitis in developing countries like Nigeria but information on the incidence and prevalence rates of consequent neurological sequelae is rare.
Objective
We herein document the prevalence of such sequelae in children following admission for pyogenic meningitis and describe associated risk factors.
Methods
We retrospectively reviewed forty-nine children managed for acute pyogenic meningitis at the Lagos University Teaching Hospital (LUTH) over a 10-year period. Information on biodata, clinical features, pre-admission treatment, investigation results, treatment, and duration of hospitalization were extracted from their case records and analysed.
Results
Thirty-two (65.3%) of these children had obvious neurological sequelae. These included neuro-motor disorders (31%), hydrocephalus (28%), hearing disorders (25%), speech and language problems (25%), recurrent seizures (22%), mental retardation (22%), visual defects (19%) and behavioural problems (3%). All ages were affected but more commonly infants. Sequelae occurred in multiples.
Children with sequelae tended to have had prior hospitalization and treatment. However no clinical or socio-economic factors showed significant relationship with the development of neurological sequelae.
Conclusions
The distribution of sequelae in our study is similar to findings of other authors but shows a higher prevalence thus further confirming the need for primary prevention of this disease and for prompt and adequate treatment of cases. We recommend early screening of survivors for sequelae so that adequate rehabilitation can be planned.
Mots clés: Afrique, méningite purulente, Nigeria, séquelles
RESUME
Introduction
Les séquelles neurologiques secondaire à une méningite bactérienne au cours de l’enfance sont fréquentes en particulier lorsque le diagnostic et le traitement sont retardés.
Objectif
Nous rapportons dans ce travail les aspects séquellaires neurologiques observés chez les enfants hospitalisés pour méningite pyogénique en relevant les facteurs de risque liés à cette affection.
Méthode
Nous avons passé en revue rétrospectivement, quarante neuf enfants traités atteints de la méningite purulante au centre hospitalier universitaire de Lagos (LUTH) au cours d’une période de 10 ans. Les informations cliniques, paracliniques et thérapeutiques tirés de leurs dossiers médicaux ont été analysés.
Résultats
Trente deux soit 65,3% des cas enfants présentaient des séquelle neurologicales : troubles neuro-moteurs (31%), hydrocéphalie (28%), troubles de l’ouie (25%), troubles du language et (25%), crise chronique (22%), retard mentale (22%), troubles visuelles (19%) et de comportement (3%). Aucun facteur clinique, socio-économique n’a pas été relevé et relié avec la survenue de séquelle neurologique.
Conclusion
Nos résutalts sont semblabes à ceux présentés par d’autres auteurs. La prévalence est élevée, impliquant ainsi la nécessité d’une politque de prévention de cette affection ainsi qu’un traitement précoce et la prise en charge des enfants porteurs de séquelles
Keywords : Africa, neurological sequelae, pyogenic meningitis
INTRODUCTION
Morbidity and mortality from bacterial meningitis remains high. (13, 5, 7, 4) While delayed presentation, partial treatment, altered sensorium and pneumococcal meningitis are known risk factors for mortality, (2, 17) those for morbidity are not as well defined. (16) Neurological sequelae constitute some of the more common complications of childhood bacterial meningitis. (3, 8) They tend to occur when there is some delay in diagnosis and treatment and such situations are not unusual in developing countries like ours.
Common neurological sequelae that have been described in childhood meningitis include problematic neuromotor development, learning disorders, ocular and visual problems, hearing loss, language and speech disorders, other cranial nerve deficits, seizure disorders, hydrocephalus and behavioural problems. (4, 3, 8, 6) Some of these sequelae occur early in the illness and are transient, whereas, others remain permanent and presumably contribute to the growing pool of children with disability in our environment.
Although subtle neurological deficits are generally found to be more prevalent than moderately severe or severe ones, Bedford et al (4) found a 10-fold increased risk of moderate or severe disability at the age of 5 years in English and Welsh children who suffered from meningitis in infancy when compared with children who did not. Such a growing load of children with disability will undoubtedly overstretch the limited resources available for childhood healthcare needs especially in resource-poor countries such as Nigeria.
The focus of meningitis studies in Africa (11, 12, 14, 15) has been on epidemiology. Other outcomes of the disease have largely been neglected. There is therefore a need to study the pattern of short and long – term neurological sequelae of children diagnosed with pyogenic meningitis. These sequelae also need to be related to presenting symptomatology and other prevailing factors at the time of diagnosis. This we believe would augment the dearth of information on the sequelae of this dreaded disease in our environment, serve as an impetus for prospective studies, as well as help in healthcare policy formulation.
METHODS
This study is a retrospective hospital based review done at the Lagos University Teaching Hospital. The case notes of all children who were discharged following admission for meningitis between January 1 1991 and December 31 2000 were retrieved.
Those with the final diagnosis of “pyogenic”, “bacterial”, or “purulent” meningitis were considered as cases while those diagnosed as tuberculous or viral meningitis were excluded from the review.
The epidemiologic and clinical data of the cases were recorded in a data entry sheet drawn up by the researchers. Significant information collected include biodata, presenting symptoms and signs, duration of symptoms prior to presentation in our hospital as well as the treatment given, cerebrospinal fluid analysis results, blood culture and serology results, serum electrolyte and urea results, antibiotic and other therapeutic agents used, as well as the duration of hospitalization. In addition, all neurological complications recorded in the case notes at the time of discharge were entered into the data entry sheets.
All data was recorded and analysed using the SPSS statistical package. Associations were tested by the chi test, while students t test was applied to continuous variables. Appropriate non-parametric tests were used where applicable. Significance was set at p<0.05.
RESULTS
Forty – nine 49 children diagnosed with acute pyogenic meningitis survived their illness. Their ages ranged between 1 day and 120 months with a median of 6 months at presentation. Majority (23) of the children were aged between 1 and 11.9 months, 11 were neonates, 9 were aged between 12 and 59.9 months while nine were 60 months and older.
Neurological sequelae were documented in 32 (65.3%) at discharge. Table I shows a breakdown of the various problems identified. They include hypertonia, hydrocephalus, speech and language disorders, hearing problems alone, seizures, mental retardation, visual defects and behavioural problems such as hyperactivity. Of the 10 children with hypertonia, 2 each had spastic quadriplegia and hemiplegia respectively. Three (9.9%) of the 6 children with visual defects were diagnosed with cortical blindness while the remaining had deficits of cranial nerves associated with the eye.
In 17/32 (53.1%) multiple deficits were documented. Neuro-motor abnormalities topped the list of sequelae whereas when combined, auditory and speech problems predominated. Generally neonates tended to have fewer sequelae but this was not statistically significant. Beyond the neonatal age group, sequelae were commoner in infancy with a two or more times tendency to occur than in other age groups, but showed no statistical significance – Figure 1.
There were no significant differences in the various risk factors assessed when children with sequelae were compared with those without. We found that children with sequelae were older at presentation, had a longer duration of treatment before arrival at the LUTH, and stayed longer in hospital although the difference was not statistically significant – Table II. There was a tendency for more of the children with sequelae to present with fever, to have had more episodes of convulsion and features of meningeal irritation – Tables III and IV. Likewise children with neurological sequelae tended to have a higher total peripheral white blood cell count, to have had a higher cerebrospinal fluid protein level, and to have required more anticonvulsant and steroid therapy – Tables V and VI.
DISCUSSION
Neurological sequelae though undesirable remain common consequences of pyogenic meningitis in childhood, a fact further borne out by this study in which 32 of 49 (65.3%) children survivors of meningitis exhibited obvious neurological sequelae at the time of discharge from hospital. The prevalence of sequelae in our group of children is a lot higher than figures reported from various studies including those from other developing countries. It more than doubles prevalence figures from Norway (12) (15.2%), Edo State, Nigeria (1) (15.5%), Jordan (7) (20%) and Ghana (6) (22%). It is also much higher than figures from India (5) (39%) and South Africa (10) (46%) thus suggesting that there may be certain peculiar risk factors in our environment. We suspect that such factors may include rampant self-medication, uncontrolled access to antibiotic use by the general populace, and poverty, all resulting in prior partial treatment and a delay in presentation for adequate care.
Several risk factors are known to influence the outcome of meningitis, chief amongst which is the aetiologic agent. An assessment of some risk factors excluding bacterial causative agents in this study showed no significant relationship between any of them and the occurrence of neurological sequelae. There was a general tendency for children with sequelae to be older on admission : have received prior treatment before arrival; require antibiotic treatment for a longer; exhibit more neurological symptoms and signs at the time of presentation; have a higher total white blood cell count; have a higher CSF protein level and a higher CSF blood sugar ratio; and require use of more anticonvulsant /steroid therapy. However, none of these factors showed a significant relationship with the development of neurological sequelae. The absence of any significant relationship between these factors and the development of neurological sequelae presumably may be a function of the small numbers of cases in the study.
Assessing the evolution of these deficits over time to determine the burden of residual deficits would have been useful but these children were lost to follow up as soon as they were discharged from hospital. This is a common finding in our environment and is a function of traditional cultural and religious beliefs where most disabilities are supposedly caused by supernatural means and as such cannot be treated by orthodox medicine.
As opposed to other studies where hearing deficits constitute the most common neurological sequelae of meningitis, disorders of tone and motor topped the list in our study. This seemingly less number of children with hearing deficits can be explained by the fact that only children who were obviously hard of hearing were identified. It is very likely that those who required special tests to diagnose their hearing loss were missed, as facilities to detect hearing loss especially in infancy did not exist in our institution then. In general however, the neurological sequelae exhibited by the survivors of meningitis in this study are similar to those described in young children admitted with bacterial meningitis in other parts of the world. Similarly, they tended to be commoner in infancy and occurred in multiples.
CONCLUSION
In conclusion, bacterial meningitis constitutes a ready source of physical and sensory disabilities in childhood and every effort must be made to ensure its prevention. While primary prevention of meningitis by immunization against the three most common causative agents of the disease viz. streptococcus pneumoniae, haemophilus influenzae and niesseria meningitides is ideal, there is need also to emphasize secondary prevention of disabilities by prompt, adequate and appropriate treatment. In addition the early recognition of disabilities is important to plan adequate rehabilitative services.
Table I: Pattern of neurological sequelae among survivors of meningitis
| Identified deficit |
No (%) |
| Hypertonia |
10 (31.3%) |
| Hydrocephalus |
9 (28.1%) |
| Speech and language disorders |
8 (25.0%) |
| Hearing problems alone |
8 (25.0%) |
| Seizures |
7 (21.9%) |
| Mental retardation |
7 (21.9%) |
| Visual defects |
6 (18.8%) |
| Behavioural problems |
1 (3.1%) |
Table II: Clinical characteristics of survivors of meningitis
| Symptoms |
Neurological sequelae present (N=32) |
Neurological sequelae absent (N=17) |
p value* |
| Age in months (SD) |
18.0 (27.6) |
16.4 (28.9) |
0.5 |
| Duration of treatment before hospitalization in days (SD) |
12.7 (13.8) |
4.7 (3.7) |
0.3 |
| Duration of hospitalization in days (SD) |
24.5 (13.7) |
22.5 (15.5) |
0.7 |
* Mann-Whitney U and Kruskall Wallis tests
Table III: Presenting symptoms among survivors with meningitis
| Symptoms |
Neurological sequelae present (N=32) |
Neurological sequelae absent (N=17) |
p value |
| Fever |
29 (93.5%) |
17 (94.4%) |
1.0 |
| Convulsion |
22 (71.0%) |
11 (61.1%) |
0.5 |
| Irritability |
9 (29.0%) |
8 (44.4%) |
0.2 |
| Headache |
3 (9.7%) |
0 (0.0%) |
0.3 |
| Rash |
1 (3.2%) |
1 (5.6%) |
0.6 |
| Photophobia |
1 (3.2%) |
0 (0.0%) |
0.6 |
| *Others |
9 (29.0%) |
6 (33.3%) |
0.7 |
* others (coma=5, vomiting=3, anorexia=2, apnoeic attacks=2, diarrhoea=1, cough=1, squint=1, neck retraction=1)
Table IV: Presenting signs among survivors with meningitis
| Signs |
Neurological sequelae present (N=32) |
Neurological sequelae absent (N=17) |
P value |
| *Seizures |
21 (67.7%) |
8 (44.4%) |
0.2 |
| Neck stiffness |
10 (32.3%) |
4 (22.2%) |
0.5 |
| Bulging anterior fontanelle |
11 (35.5%) |
3 (16.7%) |
0.2 |
| Kerning’s sign |
6 (19.4%) |
2 (11.1%) |
0.7 |
| Petechial rash |
2 (6.5%) |
0 (0.0%) |
0.5 |
* (generalised=13, partial=2, other=1, not stated=13)
Table V: Laboratory parameters of children surviving meningitis
| Symptoms |
Neurological sequelae present (N=18) |
Neurological sequelae absent (N=14) |
p value* |
| Total White cell count (SD) |
14766 (9463) |
12246 (5957) |
0.4 |
| Lymphocyte % |
33 (18) |
35 (27) |
0.7 |
| Neutrophil % |
64 (18) |
63 (27) |
0.9 |
| CSF cell count |
230 (277) |
262 (456) |
0.8 |
| CSF protein |
182.5 (162.9) |
101.7 (100.6) |
0.1 |
| CSF:Blood sugar ratio |
47.8 (26.1) |
45.5 (22.3) |
0.8 |
* Mann-Whitney U and Kruskall Wallis tests
Table VI: Treatment given to survivors with meningitis
| Treatment |
Neurological sequelae present |
Neurological sequelae absent |
P value |
| Antibiotic use |
| Penicillin + chloramphenicol or genticin |
13 |
8 |
0.55 |
| Cephalosporin ± genticin |
8 |
9 |
| Anticonvulsant use |
| Not given |
4 |
7 |
| Phenobarbitone ± any other drug |
18 |
11 |
0.27 |
| Steroid use |
| Not used |
15 |
16 |
0.7 |
| Hydrocortisone or dexamethasone |
4 |
2 |
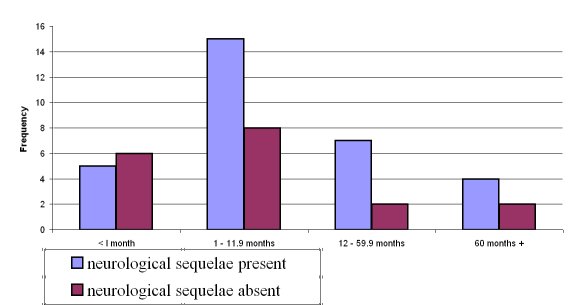
Figure 1
| COMMENT |
The authors retrospectively reviewed 49 children with acute bacterial
meningitis over a ten year period. A high proportion of the children had significant neurological sequelae ranging from weakness, sensory disturbance, hearing disorders, mental changes, recurrent seizures and
hydrocephalus. They correlated the prognosis to a number of variables. The only significant correlation was that of prior hospitalization and
treatment. They did not find a correlation between socio-economic factors or any other aspects.
- This is a highly selected population as suggested by the fact that it is a tertiary referral hospital. One therefore presumes that patients with more severe illness were referred to the unit. This therefore does not give a true reflection of the complication rate in the community.
- The numbers are relatively small compared to the period of collection ie. only 49 cases seen over a ten year period. This again may reflect the fact that the patients were at a tertiary centre.
- An important omission in this paper is the actual organisms that were responsible for the meningitis in these patients. Clearly the different bacterial organisms have different prognoses.
- This study probably does not give a true reflection of the prevalence and range of neurological complications in patients with bacterial meningitis.
Prof AHMED I BHIGJEE
Subdepartment of Neurology
The Nelson R Mandela School of Medicine
Durban
South Africa |
REFERENCES
- AKPEDE GO, DAWODU SO, UMOFFIA ME, IYASERE GEA, UKPOMWAN EMA. A Hospital-based Study of Pyogenic Meningitis in Children in Small Urban and Rural Areas of Edo State. Nig J Paediatr 2000;27:54-63.
- AKPEDE GO, IGHOGBOJA SI. The contribution of delayed Diagnosis to the Outcome in Pyogenic Meningitis. Nig J Paediatr 1996;23:4-10.
- ANTTILA M. Clinical Criteria for Estimating Recovery from Childhood Bacterial Meningitis. Acta Paediatr 1994;83:63-7.
- BEDFORD H, DE LOUVOIS J, HALKET S, PECKHAM C, HURLEY R, HARVEY D. Meningitis in Infancy in England and Wales: follow up at age 5 years. BMJ 2001;323:533-536.
- CHINCHANKAR N, MANE M, BHAVE S, BASU N, BAPAT S, BAVDEKAR A et al. Diagnosis and Outcome of Acute Bacterial Meningitis in Early Childhood. Indian Paediatr 2002;39:914-921.
- COMMEY JOO, RODRIGUES OP, AKITA FA, NEWMAN M. Bacterial Meningitis in Children in Southern Ghana. East Afr Med J 1994;71:113-117.
- DAOUD AS, AL-SHEYYAB M, BATCHOUN RG, RAWASHDEH MO, NUSSAIR MM, PUGH RN. Bacterial Meningitis: Still a cause of High Mortality and Severe Neurological Morbidity in Childhood. J Trop Paediatr 1995;41:308-310.
- GEDLU E, RAHLENBECK SI. Pyogenic Meningitis in Children in Northwestern Ethiopia. Ann Trop Paediatr 1995;15:243-247.
- GOETGHEBUER T, WEST TE, WERMENBOL V, CADBURY AE, MILLIGAN P, LLYOD-EVANS N et al. Outcome of Meningitis caused by Streptococcus Pneumoniae and Haemophilus Influenzae type b in Children in the Gambia. Trop Med Inter Health 2000;5:207-213.
- GROBLER AC, HAY IT. Bacterial Meningitis in Children at Kalafong Hospital, 1990-1995. S Afr Med J 1997;87:1052-1054.
- JOHNSON WBR, ADEDOYIN OT, ABDULKARIM AA, OLANREWAJU WI. Bacterial Pathogens and Outcome Determinants of Childhood Pyogenic Meningitis in Ilorin, Nigeria. Afr J Med Sci 2001;30:295-303.
- KAARESEN PI, FLAEGSTAD T. Prognostic Factors in Childhood Bacterial Meningitis. Acta Paediatr 1995;84:873-878.
- OKOROMA EO, IZUORA GI. Bacterial Meningitis in Children at Enugu. Nig J Paediatr 1986;13:35-40.
- PALMER A, WEBER M, BOJANG K, MCKAY T, and ADEGBOLA R. Acute Bacterial Meningitis in the Gambia: A Four-year Review of Paediatric Hospital Admissions. J Trop Paediatr 1999;45:51-53.
- PELTOLA H. Burden of Meningitis and Other Severe Bacterial Infections of Children in Africa: Implications for Prevention. Clinical Infectious Diseases 2001;31:64-75.
- PFISTER HW, FEIDEN W, EINHAUPL KM. Spectrum of Complications during Bacterial Meningitis in Adults. Results of a Prospective Clinical Study. Arch Neurol 1993;50:575-581.
- RICHARDSON MP, REID A, TARLOW MJ, RUDD PT. Hearing Loss during Bacterial Meningitis. Arch Dis Child 1997;76:134-138.

