
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
CLINICAL STUDIES / ETUDES CLINIQUES
NEUROSYPHILIS: A CLINICO- RADIOLOGICAL STUDY
NEUROSYPHILIS: ETUDE RADIO-CLINIQUE
E-Mail Contact - PATEL Vinod Bhagu :
vinodpat@ialch.co.za
ABSTRACT Purpose Method Results Conclusion Key words: HIV, Neurosyphilis, Radiology. RESUME Objectif Methode Resultat Conclusion INTRODUCTION Neurosyphilis is an uncommon condition. The ubiquitous use of penicillin for nearly a century and the community clinic based initiatives for treating sexually transmitted disease have significantly reduced the number of missed or untreated patient with syphilis. Recent estimates from the world health organization suggest that there are 350 million adults infected with treponema pallidum and two thirds are in sub-Saharan Africa and Asia [16]. Referring to sub-Saharan Africa in particular, prevalence varies from country to country. It is 2,3 % in Zimbabwe, 6% in Tanzania, 9% in Ethiopia, 12% in Mozambique and 1,6 % in South Africa. Despite the prevalent HIV epidemic the prevalence in South Africa has declined [10, 11]. It is known that the transmission of HIV may be enhanced by primary syphilis [6, 9, 29], however the converse may not be true. Whether the HIV epidemic will influence the pathological progression of syphilis to a more aggressive course is yet to be confirmed [16, 13]. The rate for the development of neuro-syphilis is not known. In a retrospective review of autopsies including 4000 patients over the age of 20, 9.7% had evidence of syphilis of whom 8% had neurosyphilis [24]. Some authors suggest that given the immunocompromised state in HIV positive individuals, syphilis may behave in a more aggressive fashion with a greater likelihood of CNS manifestations [22, 8, 20]. The clinical aspects of neuro-syphilis have been well described over many years and our experience is similar, however, the description of the neuroradiology of neurosyphilis is limited to three case series and a few case reports [3, 5, 26, 31, 32]. Sethi et al published a series of 25 cases based on clinical presentation CSF changes and confirmatory serology. Twenty one of their patients had CT or MRI of which 7 had abnormal radiological findings described as atrophy in 2, intramedullary hyperintensities of the spinal cord in 3 and stroke in 1 [25]. The largest series describing the radiological changes seen in neurosyphilis was published by Brightbill et al [3]. Their series of 35 patients included 32 patients who were HIV positive. All the patient had MRI imaging while 19 also had CT scan imaging. They described infarcts in 32%, white matter hyperintensities in 20%, gummas and meningeal enhancement in 6%, cerebral atrophy in 37% and normal scans in 31%. Their series did not include spinal cord imaging. We describe the clinical presentations and radiolological findings seen in our unit, thus expanding the described population for neurosyphilitic radio – diagnosis. METHOD A retrospective chart review was done on all patients classified and managed as neurosyphilis, at our neurological unit over a period extending from 1994 to 2005. Inclusion criteria: 1. Patients with a neurological presentation with positive serum serology suggestive of syphilis, Wasserman reaction (WR or VDRL) together with an abnormal cerebrospinal fluid (CSF) suggestive of inflammation, a positive CSF VDRL (not essential). All patients also required a positive confirmatory test such as fluorescent treponemal antigen (FTA) or treponema pallidum hemagglutinin assay (TPHA). Alternative aetiologies were excluded. The following data was extracted and entered onto an excel spread sheet for analysis: Cellular, biochemical CSF findings and response to treatment were compared between HIV positive and HIV negative patients with neurosyphilis using the non parametric wilcoxin rank sum test. The rank sign test was used to compare CSF changes between the initial and follow up CSF. RESULTS Fifty-three patients were identified, however only forty-one charts were available for review. Neuroradiological findings were available on 39 patients. Two patients had no imaging; one who presented with a polyneuropathy was incidentally discovered to have neurosyphilis having satisfied the inclusion criteria and another who presented with a polyradiculopathy. There were 25 males and 16 females with the mean age being 35 + 11.1. The clinical presentations were classified as mentioned in the method. The clinical as well as the radiological findings are demonstrated in table 2. Imaging findings are shown in 3 patients. Patient 1 has several meningeal based mass lesions, which resolved completely on therapy, patient 2 has cranial nerve enhancement and patient 3 with spinal cord changes. Patients 1 and 2 improved on 14 days of penicillin G at 24 million units per day. The third patient was lost to follow up. Outcome data was available on 26 patients 17 of whom improved, 7 remained the same, 1 patient’s neurological status deteriorated following hypoxic brain injury from seizures and 1 patient died. 15 patients defaulted follow up. When examining for the influence of HIV status on outcome the following emerges. The overall p value comparing the association between HIV and outcome in the following 3 categories, poor, improved and defaulted was p = 0.7. HIV positive patients are less likely to improve (36%) compared to HIV negative patients (45%). However the difference was not statistically significant (p = 0.6). As the numbers are small, there may be insufficient power to show a statistically significant difference. If one looks at the strength of association, the chances of improving if one is HIV positive are almost half that of a HIV negative person; odd ratio: 0.54 (CI 95%: 0.12 – 3.3) This must be viewed with caution because of the small numbers and the large p value. HIV positive patients are more likely to default (50%) compared to HIV negative patients (35%). However the difference was not statistically significant, p = 0.4. Again the numbers are small so there may be insufficient power to show a statistically significant difference. If one looks at the strength of association, the chances of defaulting if you are HIV positive are almost twice that if you are HIV negative; odds ratio 1.9 (CI 95%: 0.4 – 9.3). This must be viewed with caution because of the small numbers and the large p value. There were 8 patients with repeat CSF examination, which was done after a mean of 6 weeks (range 4-12). A sign rank test was used to compare the first and second CSF values. There was no relationship between duration of symptoms and type of symptoms. In general the duration varied from a few days to several months. Only 2 patients had symptoms of 2 years and 5 years each. These patients had TD and GPI respectively. DISCUSSION Descriptions of neurosyphilis are limited to small case series. The largest series of 161 patients recently described confirmed the clinical variety of presentation. The clinical spectrum is similar to our experience [4, 27, 28, 33]. The radiological features in neurosyphilis are less well described. Our series demonstrates the non-specific imaging findings and the varied images one may see with neurosyphilis. This includes normal imaging, age inappropriate cerebral atrophy, hydrocephalus, infarcts and enhancing mass lesions that were both parenchymal and meningeal based. One needs to have a high index of suspicion and be cognizant of the varied radiological picture seen. Imaging is unlikely to be different when dealing with HIV positive patients however, one must be aware of multiple and alternate etiologies in the immunocompromised patient [3, 5, 31, 32]. The radiological manifestations of neurosyphilis are non-specific and vary with the accent of the clinical syndrome. Our results are consistent with Brightbill’s series [3] in that we had a spectrum including normal, age inappropriate atrophy, infarcts, parenchymal enhancing lesion. We differ in that they had not described any spinal or post treatment radiological manifestations. Our review had a series of patients where imaging for clinical myelitis revealed both intramedullary hyperintensity usually over a long length of the spinal cord, with or without focal intramedullary enhancing lesions. Further, pial and extradural enhancement suggestive of extradural mass lesions was seen. The recent series by Timmermans et al [33] have included myelopathy as part of their review but did not comment on the radiological findings. Our serological findings are similar to other publications [19, 30, 33]. The VDRL in the CSF was positive in 73.13% of patients. A diagnosis of neurosyphilis may also be entertained when the CSF is active with a positive blood WR and a positive TPHA or FTA-ABS, provided there is no alternate explanation for the neurological syndrome and CSF changes. In HIV positive individuals CSF finding may be due to HIV itself. Previous publications [8, 17, 18] have suggested that only a cell count greater than 20 would be attributable to neurosyphilis when the patient is also HIV positive. This is practically irrelevant when there is serological confirmation of neurosyphilis, however this may be relevant when repeat CSF is done to measure response to treatment. A clinical improvement together with a reduction in CSF VDRL titer would be good indicators of improvement. The CDC recommends a four fold decrease in the RPR/WR titer and normalization of CSF at 2 years as indicators of cure. When comparing cell counts, protein and glucose levels in CSF between HIV positive and negative patients we found no difference. We did not have CD4 counts and did not characterize the CSF cellular changes further into cell types. A recent report [19] suggests that there may be a greater frequency of B cells in CSF in HIV positive individuals with neurosyphilis. With regards to CSF examination in HIV positive individuals who are neurologically asymptomatic with primary or secondary syphilis it is suggested that if the CD4 count is less than 350 or the serum RPR is ≥ 1: 32 one should routinely do a CSF examination [17, 34]. It is controversial whether HIV has an influence on the pathologic progression of syphilis. Several authors [2, 7, 14] have alluded to a more rapid and aggressive course with earlier neurosyphilis in patients with syphilis who are HIV positive. It is well known that the spirochete can be isolated from CSF in 25% of patients with primary syphilis early in the course of syphilitic infection, regardless of HIV status. The ability of T Pallidum to spread, although uncertain, is possibly related to surface protein for tissue binding and immune evasion. Expression of surface lipoproteins VspB in animal studies has been correlated with high serum levels of Borrelia and VsP A with CNS invasion and persistence within the central nervous system [13, 30]. Expression of similar surface proteins may contribute to the virulence of T Pallidum. The pathology in meningitis is characterized by inflammatory infiltrate of lymphocytes and plasma cells and occasional polymorphonuclear leukocytes. Additionally, in meningovascular syphilis one sees fibroblastic thickening of the intima, thinning of the media and luminal narrowing which leads to vessel occlusion and ischemic infarction. When a gumma forms the histological correlate is chronic granuloma formation with multinucleate giant cells [30]. Although local figures are not available, the HIV epidemic, presently with an incidence of 40.7% in our locale would have resulted in far more patients with neurosyphilis than that seen by our unit. Lukehart et al [12] suggested that the clearance of T pallidum is impaired in HIV positive individuals. The research relating to HIV and neurosyphilis includes small numbers and no firm conclusions could be drawn. The serodiagnosis of syphilis requires a positive non-treponemal test such as RPR or VDRL and a confirmatory test TPHA or FTA-ABS. In the HIV positive individual the RPR may be negative attributed to the prozone phenomenon. Two patients in this series demonstrated this phenomenon where the initial screening test was negative but became positive on a subsequent test. The prozone phenomenon; due to an overwhelming antigen-antibody complex lattice, although real is uncommon and the frequency unknown. Nevertheless one should be aware of this and maintain a high index of suspicion in the appropriate patients and repeat the test if initially negative [12, 30]. Further it is well documented that false positive rates for RPR are greater in HIV positive patients being 1% to 5.8% when compared to HIV negative patients; 0,2% to 0,8% [1, 23, 25]. Therefore in an environment where HIV is prevalent it is imperative to confirm neurosyphilis with a specific confirmatory test Therapy would be similar in HIV positive and HIV negative individuals, however the drop out rate may be greater in HIV positive patients [21]. The currently recommended therapy is aqueous crystalline penicillin G 12 to 24 million units daily given 4 hourly for 10 to 14 days. There is little data on alternatives but these include long term, 30 days of tetracycline at 2g per day or doxycycline at 400mg per day. The goal of therapy is to halt progression and response is best seen in the meningeal type of neurosyphilis. The late stages such as GPI or TD where pre-existing damage is present, would not necessarily reverse. Marra et al have suggested that if a CSF VDRL of more than 1:1 is predictive a five times likelihood of non-response in terms of normalization of CSF VDRL [18]. It is expected that if CSF VDRL and white cell count normalizes by 1 year, the protein is slow to normalize. Our experience has shown response within several months in both the cell count and the protein level, however this should be viewed with caution as our numbers are small. CONCLUSION Neurosyphilis remains an uncommon condition even in an environment where the HIV epidemic is at its peak. One needs to maintain a high index of suspicion. In HIV positive individuals who are neurologically asymptomatic, with a RPR of greater than 1:32 or a CD4 count of less than 350 consider doing a CSF examination. The radiology of neurosyphilis is non-specific and includes meningeal and extradural mass lesion, parenchymal enhancing lesions, infarcts, age inappropriate atrophy, and intramedullay hyperintensities within the spinal cord. There is no difference in CSF changes between HIV seropositive and HIV seronegative individuals who have neurosyphilis. Therapy would be similar regardless of HIV status but follow up may be longer. Table 1: Comparison of CSF changes between HIV positive and negative patients
Table 2: Summary of clinical and radiological findings
 Figure 1 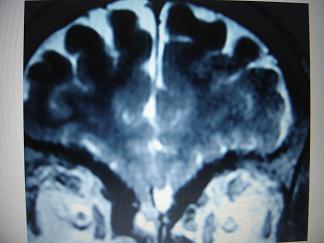 Figure 2  Figure 3 REFERENCES
|
© 2002-2018 African Journal of Neurological Sciences.
All rights reserved. Terms of use.
Tous droits réservés. Termes d'Utilisation.
ISSN: 1992-2647