
|
|
|
CASE REPORT / CAS CLINIQUE
PHARYNGEAL-CERVICAL-BRACHIAL SYNDROME: AN ATYPICAL CASE OF GUILLAIN-BARRE SYNDROME WITH ANTI-SULFATIDE IGM ANTIBODIES
SYNDROME PHARYNGO-CERVICO-BRACHIAL : UN CAS ATYPIQUE DE SYNDROME DE GUILLAIN-BARRE AVEC DES ANTICORPS ANTI-SULFATIDE IGM
E-Mail Contact - FOTSO Vicky :
fotso21@gmail.com
ABSTRACT Pharyngeal-cervical-brachial (PCB) syndrome, a rare form of Guillain-Barre syndrome (GBS) is defined by rapidly progressive oropharyngeal and cervicobrachial weakness associated with areflexia in the upper limbs. We report the observation of a 30-year-old adult male who presented, 10 days after acute diarrhea, oculomotor and severe bulbar palsy followed by cervicobrachial diparesis with hyporeflexia in the upper limbs. Cerebral magnetic resonance imaging (MRI), the otorhinolaryngological examination, the cerebrospinal fluid (CSF) analysis, the prostigmine test were normal as was the electromyography (EMG) performed on day 10. Laboratory test results such as antiacetylcholin receptor antibodies were negative. At the EMG performed on day 18, there were some signs of demyelination; the diagnosis of PCB syndrome was made. Determination of antiganglioside antibodies revealed positive Anti-sulfatide IgM antibodies. After administration of immunoglobulin therapy, symptoms got better with a complete recovery in 6 weeks. Keywords: Ophthalmoparesis, Anti-sulfatide IgM antibodies, Guillain-Barre syndrome, Pharyngeal-Cervical-Brachial syndrome. INTRODUCTION GBS is a heterogeneous group of related disorders, which shares a common pathogenesis and several clinical features, including: an infection history, monophasic disease course and symmetrical cranial or limb weakness (10). His PCB variant is a rare entity not known by most neurologists. Therefore, patients presenting with PCB are initially misdiagnosed as having diphtheria, brainstem stroke, myasthenia gravis (MG) or botulism (3,7,11). A history of campylobacter jejuni diarrhea is frequent. The study of nerve conduction suggests, often, an axonal more than a demyelinating mechanism. Nevertheless, an EMG done early can be normal without eliminating the diagnosis (3). PCB syndrome is frequently associated with the presence of antigangliosides anti-GT1a and possibly anti-GQ1b. Although PCB variant of GBS is rare, it should always be considered in patients presenting to the emergency room with symptoms and signs suggesting progressive brainstem dysfunction such as atypical multiple cranial nerve palsy with normal cerebral MRI (10). We report a case of PCB syndrome in a 30-year-old male; presenting with descending oculopharyngeal and cervicobrachial impairment who was initially misdiagnosed as having a brainstem lesion, botulism or myasthenia gravis, thus highlighting the diagnostic difficulty in an atypical presentation of GBS. PATIENT AND OBSERVATION A 30-year-old male patient presented at the emergency room with horizontal diplopia and upper limbs weakness, which occurred 10 days after an acute diarrhea. Seven days later, he developed dysarthria, dysphagia with outflow of liquids through the nose and chewing disorder. He was admitted to our department at day 14. The initial examination found a conscious patient without any sign of meningeal irritation. He had dysarthria, dysphonia, severe dysphagia for solid and liquid food, bilateral facial palsy, diplopia with ophthalmoplegia, (Fig 1A, B, C, D) and preserved photomotor reflex without any sensory disturbance. Manual muscle testing revealed a -3/5 weakness in the cervical muscles and a -4/5 weakness in the shoulder and proximal upper limbs muscles; muscles of lower limbs were normal. At deep tendon reflexes testing, we found hyporeflexia in upper limbs, normal in lower limbs. In addition, he had dysautonomic disorders such as constipation, tachycardia and hypersudation. Brain MRI, otorhinolaryngological examination with cavum exploration did not show any abnormal lesion. The prostigmine test was normal as was the EMG performed at day 10. The anti-RACH and anti-MUSK antibodies were negative. CSF analysis found no sign of infection nor albuminocytological dissociation. The repetitive nerve stimulation study (RNS) did not show a significant decrement of the compound muscle action potentials (CMAPs). There was an elongation of distal latencies with a decrease in the amplitude of motor potential at the EMG performed on day 18 (Fig 2). Determination of antiganglioside antibodies revealed positive Anti-sulfatide IgM antibodies. The patient was diagnosed having PCB variant of GBS, and we treated him with immunoglobulin. He received daily intravenous immunoglobulin, 400mg/kg/day, for 5 days. His symptoms improved a week afterwards with complete recovery in 6 weeks (Fig 3A, B, C, D). DISCUSSION PCB syndrome was first described as a regional variant of GBS in three cases reported by Ropper in1986. It’s a rapidly progressive oropharyngeal, neck and shoulder weakness, in the absence of sensory disturbance and with relative sparing of the lower limbs (6,9,11). In many clinical reports, the presence of additional features indicates an overlap with other GBS variants: ataxia with ophthalmoplegia suggests an overlap with Fischer syndrome (FS) (5,12); and if in addition consciousness is impaired, an overlap with Bickerstaff brainstem encephalitis (BBE) is suggested (9,11). The existence of PCB with overlapping FS and BBE provides clinical evidence that PCB and these conditions form a continuous spectrum (6). Many clinical forms of PCB are described in the literature; patients presenting with rapidly progressive oropharyngeal and cervicobrachial weakness associated with hyporeflexia or areflexia in the absence of ophthalmoplegia or leg weakness are defined as ‘pure’ PCB (6,11). On the other hand, those who also displayed normal or exaggerated reflexes, which is apparent in about 10% of GBS are defined as having ‘PCB with preserved muscle stretch reflexes’ (3,6,9,11). Incomplete forms of PCB syndrome have also been described: patients without arm and neck weakness have « acute oropharyngeal palsy »; patients without pharyngeal palsy have « acute cervicobrachial weakness », « fulminant PCB weakness » is when patient develop early and severe leg weakness (9). The findings of a prospective study of more than 250 patients with GBS done at the Massachusetts General Hospital showed that FS (5%) was the most frequent GBS variant with PCB (3%) as the second (9,11). In the largest case series which examined 100 patients with PCB and its overlap syndromes in Japan, a history of upper respiratory tract infectious symptoms and diarrhea were observed in 71% and 30% of patients respectively. Similar to GBS, 31% had serological evidence of Campylobacter jejuni infection (6,11). There are also non-infective associations reported: various vaccines, autoimmune diseases, immunosuppressive drugs, and surgery (10). Our patient had a history of diarrhea 10 days before symptoms’ onset, but Campylobacter jejuni serology wasn’t performed. In patients with PCB syndrome, some leg weakness may be present, but oropharyngeal, neck and arm weakness should be more prominent (9). Some patients may also develop additional facial weakness or ophthalmoplegia (6,9,12). In the 100 case series of PCB of nagashima and al; the most frequent initial symptom was arm weakness (29.0%), followed by dysphagia (17.0%) and diplopia (17.0%) (3,6). Predominant arm weakness was proximal in 47.0% and distal in 28.0%. Autonomic dysfunction occurred in 20.0% of cases (6). Our patient consulted initially for diplopia otherwise he had dysautonomia and bilateral facial weakness. The diagnosis of PCB can be challenging in early disease stage. Patients are initially misdiagnosed as having brainstem stroke, MG or botulism (3,4,9,11), diphtheria, brainstem tumor, neurobehcet disease, multiple sclerosis (6,10). Otherwise, some case reports have described the comorbidity of MG and GBS (8). Brainstem ischemia should be considered if symptoms start abruptly, while fluctuations in weakness or obvious fatigability are more suggestive of MG. Botulism is also characterised by ptosis, internal and external ophthalmoplegia, bulbofacial weakness and symmetrical descending flaccid paralysis (3,11). However, MG and botulism can usually be excluded in the presence of sensory deficits or absent reflexes; if doubt persists low and high frequency repetitive nerve stimulation and triple stimulation technique can be used to assess patients with MG and botulism respectively; particularly in the early stages when electrodiagnostic criteria for axonal GBS do not fit the clinical criteria (7). Differential diagnoses were discussed in our case; mainly brainstem stroke, MG or botulism; because of atypical presentation. Therefore, many investigations have been performed uselessly. The neurophysiological findings in PCB are axonal rather than demyelinating. It can be normal in early disease stage. In that case, repeated testing after few days is advised (3,11). When comparing anti-ganglioside antibody–mediated GBS, PCB is considered a nodo-paranodopathy, because reversible conduction failure has been detected by serial conduction studies in the distal and intermediate motor nerve segments (2,7). The most important early investigation is brain MRI to exclude brainstem ischemia, inflammation or brain tumours, which may also involve the skull base or meninges (3,10,11). Normal CSF and neurophysiological testing do not exclude PCB in early disease stage (3,11). Therefore, we should not delay diagnosis and treatment if GBS or its variants are suspected on clinical grounds (9). MRI and CSF analysis were normal in our case. The strongest argument for PCB diagnosis is the presence of anti-GT1a-IgG antibodies (3,6,11). Some of which might cross-react with GQ1b (9). Patients with anti-GT1a antibodies develop PCB because a GT1a ganglioside, in addition to being abundantly detected at the cranial nerves such as the glossopharyngeal and vagus nerves (4), is also present in the oculomotor nerves (11). In 100 patients with PCB, Anti-GT1a IgG and Anti-GQ1b IgG antibodies were positive in 51.0% and 39.0% of the patients respectively (6). Increased titers of immunoglobulin M (IgM) antibodies to sulfatide have often been reported in patients with immune mediated neuropathies. But a selective reactivity to sulfatide, however, is rarely found and is associated with different forms of neuropathies, limiting its usefulness in the diagnosis of these latter (1). Determination of antiganglioside antibodies in our patient revealed positive Anti-sulfatide IgM antibodies but we did not know how to interpret this result because Anti-GT1a IgG and Anti-GQ1b IgG antibodies that we expected to find were negative. The general principle of GBS management includes the use of intravenous immunoglobulin or plasma exchange. In addition, nasogastric feeding and ventilator support can be useful if bulbar and respiratory functions are impaired (11). It has been reported that the ptosis as well as dysphagia associated with the PCB variant can be improved by administration of acetylcholine esterase inhibitor (4). In the largest series, patients with pure PCB were more likely to require intubation than those with overlap syndromes and this correlated with degree of bulbar involvement (11). Our patient was not intubated and his symptoms were improved after immunoglobulin therapy with complete recovery after 6 weeks. CONCLUSION The PCB syndrome is a regional axonal variant of GBS not known by most neurologists. Our case, whose clinical presentation was misleading initially, highlights the diagnosis difficulty of this entity. However, the recognition of this syndrome based on core clinical features (history of infection, monophasic disease course, and symmetrical cranial or limb weakness) is important to establish early correct diagnosis, avoid unnecessary investigations and guide appropriate use of immunotherapy (10). CONSENT FOR PUBLICATION: The patient gave a written consent for publication. AUTHOR ROLES: Hicham El Otmani and Vicky Fotso worked at the Conception and writing of the manuscript. Mohamed abdoh Rafai and Bouchra Moutawakil : have read and criticized the manuscript. All authors read and approved the final manuscript. COMPETING INTERESTS The authors declare no competing interest. 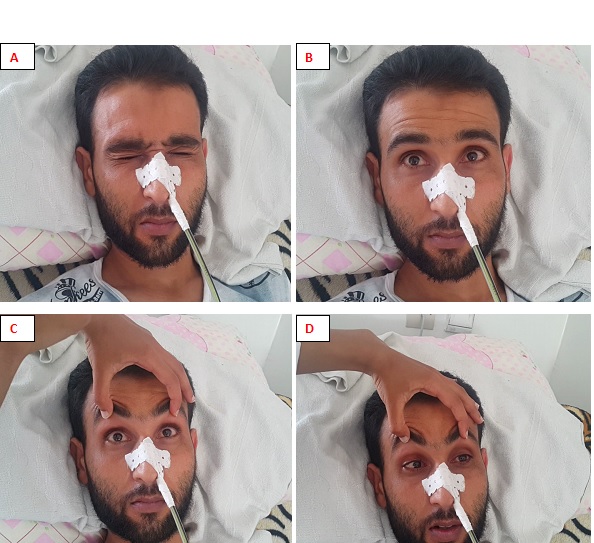 Fig 1: patient at admission: the oculomotor nerves III and VI as well as the VII nerve are impaired; A : mild bilateral facial palsy ; B: left internal strabismus (paresis of left VI nerve) C : gaze upward paralysis (bilateral III nerve) D : left eye abduction paresis.
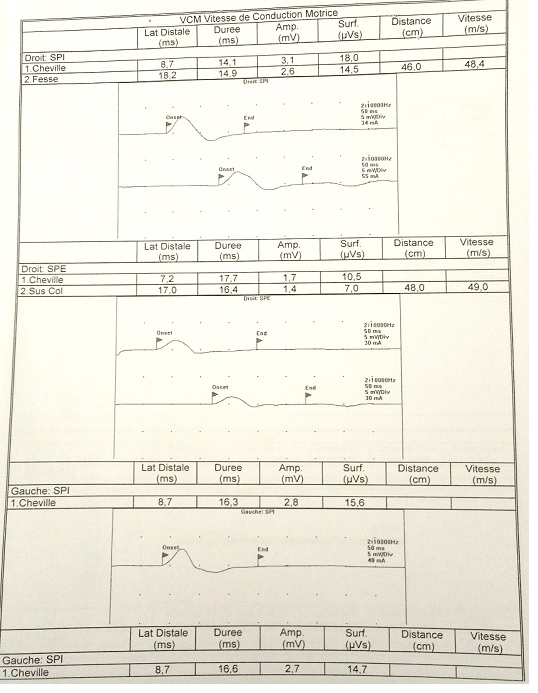 Fig 2: EMG showing an elongation of distal latencies with a decrease in the amplitude of motor potential in lower limbs.
 Fig 3: patient 6 weeks later: cranial nerves examination is normal; A: eyelid orbicularis muscle (VII nerve); B: right VI nerve and left III nerve; C: III nerve ; D: left VI nerve and right III nerve. REFERENCES
|
© 2002-2018 African Journal of Neurological Sciences.
All rights reserved. Terms of use.
Tous droits réservés. Termes d'Utilisation.
ISSN: 1992-2647