NEUROEPIDEMIOLOGY / NEUROEPIDEMIOLOGIE
SIAPEM – BRAZILIAN SOFTWARE DATABASE FOR MULTIPLE SCLEROSIS RESEARCH IN TROPICAL COUNTRIES
- Universidade do Rio de Janeiro (UNIRIO), Brazil
- Santa Casa de Misericórdia, São Paulo, Brazil
- Harvard Medical School, Boston, USA
- Hospital da Lagoa, Rio de Janeiro, Brazil
E-Mail Contact - PAPAIS ALVARENGA Regina Maria :
ABSTRACT
SIAPEM is a software conceived and developed by the Group of Neuroimmunology from the Brazilian Academy of Neurology (1990/1998), affiliated with the World Federation of Neurology. SIAPEM is the protocol being used as a research tool in the multicentric study named “Projeto Atlântico Sul” (South Atlantic Project) that will allow for uniformity in data collection and analysis of MS in Latin America countries. Record standardization will render possible, for the first time, the clinical developmental profile of MS in South America, the selection of some cases for research projects with specific goals, studies of MS control-group population, and for projects where the culling of a critical number of patients depends on the cooperation of diverse centers.
SIAPEM was structured in five stages to gather clinical, epidemiological and developmental data from patients with multiple sclerosis diagnosed according to POSER criteria, plus information derived from paired controls. Running on ACCESS program, this is a software data bank that allows entries for immediate analysis of results, regardless the number of patients, with a simple and effective layout. It is a time-saving process providing a uniform approach for individual cases, featuring Windows for international standard data records regarding classification, macro- and microenvironmental factors and laboratory data. It runs on PCs and is a flexible system that may be customized to meet the user’s needs.
Keywords: Multiple Sclerosis, databasing, multicenter studies, multiple sclerosis, multiple sclerosis in South America, multiple sclerosis epidemiology, Esclerosis multiple
RESUMEN
Fundamento
« SIAPEM es un software concebido y desarrollado por el Grupo de Neuroinmunología de la Academia de Neurología de Brasil (1990/1998) que está afiliada con la Federación Mundial de Neurología.SIAPEM es el protocolo que se utiliza como una herramienta de investigación en el estudio multicéntrico que se denominó : « Proyecto Atlántico Sur » y que permitirá uniformizar la recolección de datos e investigación sobre la Esclerosis Múltiple en los países de América Latina. Esta normalización hará posible, por primera vez, el perfil de la presentación clínica de la Esclerosis Múltiple en América del Sur, la selección de algunos casos para los proyectos de investigación con metas específicas, los estudios de población casos-control de Esclerosis Múltiple , y para proyectos donde la selección de un número crítico de pacientes depende de la cooperación de centros diversos.
Métodos
SIAPEM se estructurará en cinco pasos para la recolección de los datos clínicos, epidemiológicos y de evolución de la enfermedad en los pacientes con Esclerosis Múltiple diagnosticada según el criterio de Poser, junto con información derivada de los centros asociados.
Resultados
Se utiliza con el programa ACCESS 2000, y es un banco de datos de software que permite obtener resultados en forma inmediata de los datos entrados, no importando el número de pacientes existente, con un diseño simple y práctico.
Conclusiones
Es un proceso que ahorra tiempo y permite mantener una perspectiva uniforme de los casos individuales, mientras que ofrece un ambiente Windows para procesar los datos normales internacionales con referencia a la clasificación, los factores macro-micro-medio ambientales y los datos del laboratorio. El programa se utiliza en Computadoras personales tipo PC y es un sistema flexible que puede personalizarse de manera de satisfacer las necesidades del usuario.
Keywords: esclerosis multiple en America Latina, esclerosis multiple e estudio multicéntrico, epidemiologia de la esclerosis multiple
Corresponding Author: Claudia Maria Miranda Santos
Av Pres Kennedy 999 sala 204 – Centro – Duque de Caxias
Brazil – CEP 25010 000
Claudia_Santos@yahoo.com
Presented in part at American Academy of Neurology – Annual Meeting of 2001
INTRODUCTION
At the end of the last century the gold pattern of Multiple Sclerosis studies was the development of computerized protocols [1][2][3][4]. SIAPEM – system for evaluation of protocols in multiple sclerosis [5], is the database created in Brazil and applied as the main tool of a Brazilian multicentric research, the South Atlantic Project [6], in some countries of Latin America and in collaborative studies with Spain (Malaga) and France (Limoges).
SIAPEM SYSTEM: BASIC CONCEPTS
SIAPEM is a system developed on standard databank ACCESS 2.0 software. It runs on personal microcomputers IBM-PC and compatibles, with a 4-megabyte RAM memory, an 2-gigabyte-harddisk, and a high definition color monitor. Besides the standard operating system (MS-DOS), MS-WINDOWS is also required.
SIAPEM is a database to be adopted in neurological reference centers for treatment and monitoring of patients with definite MS, according to Poser criteria [7]. Records include patients’ full name, date of birth and name of the research center. These are deemed necessary in all research centers prone to use the SIAPEM system so as to avoid multiple entries referring to the same patient. The name entry should be coupled with security measures to keep all data confidential, according to internetional rules and regulations. Confidentiality of patient’s identity is strictly maintained. Patient’s names and records will be promptly deleted when a duplication occurs. It is strongly recommended that the microcomputer that holds patients’ data should be properly protected.
SIAPEM FORMAT
General Structure
SIAPEM was structured in 5 fundamental stages that comprise, in a detailed and systematized way, information regarding the patient, his antecedents, clinical and epidemiological history, complementary investigation and clinical characteristics of attacks, analyzed according to Kurtzke’s functional systems [8]. SIAPEM database (figure 1) was designed to be used “by experienced and competent neurologists for the diagnosis of this disease” [7].
Stage 1 – Justification for the patient’s enrollment in the study (figure 2)
Stage 1, labeled GENERAL INFORMATION has an initial segment – a summaryc specifying the number of attacks, clinical evidence and findings from complementary tests directed at MS diagnosis, enough to justify patient’s enrollment. Accomplishment of this stage will entirely depend on the neurologist who, after analyzing all information at hand about that patient, defined him as definite or probable multiple sclerosis. The number and dates of previous attacks, main neurological manifestations and MS clinical course (relapsing-remitting or primary progressive) must be indicated. Terms used in SIAPEM were taken from literature [7]: Relapsing and remitting – at least a period of 1 month between attacks is demanding to define this form; Primary Progressive – since the onset, the development of MS is progressive and after a minimum of 6 months another SNC dysfunction is verified via clinical evidence (new neurological manifestations – signs or symptoms) or paraclinical evidence (demyelinating lesions of white matter on MRI, although clinically asymptomatic), thus configuring the spatial and
temporal evolution of MS. When filling in the SIAPEM protocol for patient in the progressive category, each new neurological manifestation that constitutes an attack (lasting more than 24 hours) must be entered as new attack. For progressive patients, tumors, infections and degenerative diseases must be ruled out. Tests for HTLV-I and HIV are mandatory in tropical countries.
Skin color, eyes color and birthplace of the probing patient, their family antecedents (including great-grandparents) are recorded. The methodology used was the same as utilized by Brazilian Institute of Geography and Statistics for the skin color survey census: basic colors (for pure types): white (Caucasian), black, yellow (oriental), red (American indian) and mulattos (in all shades and combinations). Eyes colors: blue, green, light or dark brown. Birthplace: city, region or country.
Personal antecedents consist of data referring to: infections, vaccinations, head or spine traumas; pathological antecedents focuses on the presence of other immune, vascular or rheumatic diseases; family antecedents records the occurrence of cases of MS within the family. Patient’s enrollment in the protocol is completed by the assessment of the current neurologic status including a specialized neuro-ophthalmological examination (figure 3). Such data will be the basis for grading the patient at the minimal record of disabillity [9] by functional systems (FS), expanded disability status scale (EDSS) and IFMS.
Stage 2 -Information about previous attacks (figure 4)
Analysis of each previous attack consists of: prior events (infectious diseases, pregnancies, surgeries, etc) within a period of 3 months before; nonspecific symptoms (fatigue, headache, etc); and neurological manifestations observed during the attack (according to the functional systems). Individual limbs must be specified when appropriate in the various functional systems (FS). Whenever possible, is required to fulfill the functional systems scale. Any manifestation not covered by the FS scale should be included in the item – Others (FS-O). Information source may be collected from clinical history or previous medical records. Under neurological manifestations of previous attacks, new and old signs and symptoms must be entered, the latter consisting of recurrences of a new attack. In the case of different degrees of dysfunction within a given attack, select the worst, for example: the patient was monoplegic at onset but became tetraplegic, enter tetraplegia as the pyramidal manifestation for that attack.
Stage 3 – Information about attacks after enrollment in the study
After the patient’s enrollment in the study each subsequent attack must be separately entered in the SIAPEM. These data include type of therapy indicated, type of onset (acute, subacute or insidious), indication of the preceding events, nonspecific symptoms, transitory symptoms with less-than-24-hour duration, the neurological manifestations of the attack by means of Functional Systems (FS), clinical history and all clinical evidence. Information on complementary investigation and treatment are also included.
Stage 4 – Periodic neurological assessment (figure 5)
Periodic checking of the patient, at least once a year, follows the same guideline described in the enrollment stage: analysis of alterations through the minimal record of disability.
Stage 5 – Information about paired controls (figure 6)
The same questionnaire about risk factors is applied for paired controls: individuals with the same birthplace, gender, skin color and same place of residence up to adolescence. Relatives are not accepted for that purpose, being ideal a neighbor or schoolmate with no neurological manifestation attributable to a primary central demyelinating disease.
CONCLUSIONS
SIAPEM was released in final version in 1995 . It is written in Portuguese and applied in the South Atlantic Project. Demographic, clinical and laboratorial information on 602 MS patients (1/3, mullatos or blacks) were collected according to a unique methodology since 1998 [6]. It is one of the most complete and reliable files on the natural history of the MS in Brazilians, a population derived from a tropical area and highly endowed with miscegenation. The well spread and well-known relationship between MS and geographical latitude is being put aside. Nowadays, the ethnic background of the studied population has been emphasized [10][11]. SIAPEM was a vital tool to the MS studies in tropical areas because it supplied parameters in order to minimize the misdiagnosis and drove to a better selection of the studied population. The data base focuses the accuracy of the diagnosis, propitiates the study of the frequency and severity of the attacks and opens the possibility of development of case-controls and prospective studies. Some deficiencies of SIAPEM were noted [12]: not to be available for the internet; page-topage design; to describe previous and subsequent attacks in different ways hiding analysis and to be more descriptive than analytical system. The SIAPEM new version , already under development, will focus on these points. SIAPEM can be understood as a very important database to be employed in the study of multiple sclerosis in tropical countries.
| Agradecimiento |
| We sincerely thank to Mrs. Magdalena Oehninger, who kindly helped us with the resumen. |
Figure 1 – First Page of SIAPEM
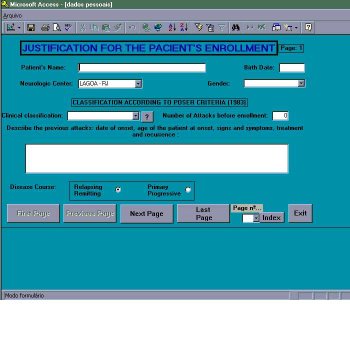
Figure 2 – Module of inclusion : Justification for the patient enrollment.
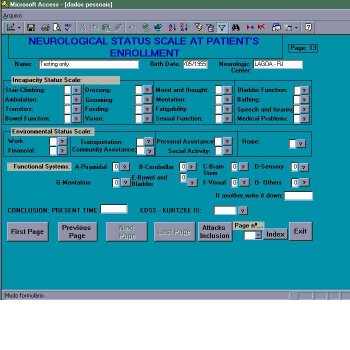
Figure 3 – Module of inclusion – Neurological status scale at patient’s enrollment.
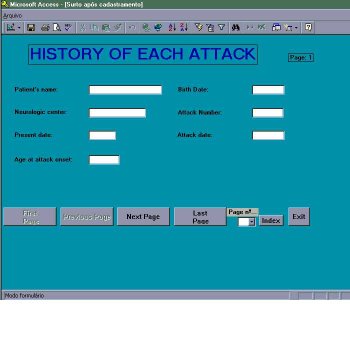
Figure 4 – Module of Inclusion – Information on attacks
Figure 5 – Module of Inclusion – periodic neurological assessment.
Figure 6 – Module of Inclusion – Risk Factors (case-control)
REFERENCES
- PATY DW. MS-COSTAR : a computerized patient record adapted for clinical research purposes. Satellite Symposium, Victoria, Canada. Annals of Multiple Sclerosis 1993; A-29 (Abstract) .
- CONFAVREUX C, COMPSTON DAS, HOMMES OR, McDONALD WI, THOMPSON AJ. EDMUS , a European data base for multiple sclerosis. J Neurol Neuros Psyc 1992;55:671-676.
- CONFAVREUX C et al. Current status of computerization of multiple sclerosis clinical data for research in Europe and North America: the EDMUS/MSCOSTAR connection. European Database for Multiple Sclerosis. Multiple Sclerosis-Computed Stored Ambulatory Record. Neurology 1995;45(3 Pt 1):573-576.
- MARKUS S. MUSIS 2.0-Multiple Sclerosis Information System: An easy-to-use database to improve the care of patients with multiple sclerosis. MultScler1999;5(4):299-301.
- ALVARENGA RMP, SANTOS CMM. SIAPEM – a Brazilian database for multiplesclerosis In:LACTRIMS, Buenos Aires. Revista Neurologica Argentina
2000;25: 49 -49. Abstract.
- PAPAIS-ALVARENGA RM, MIRANDA-SANTOS CM, ALVES SV, TILBERY CP – Esclerose múltipla: projeto de pesquisa para análise do perfil clínico e evolutivo da EM no Brasil (Projeto Atlântico-Sul). Rev Bras Neurol 1995;31(2):51-59.
- POSER CM, PATY DW, SCHEINBERG L, McDONALD I, DAVIS FA, EBERS GC, JOHNSON KP, SIBLEY WA, SILVERBERG DH, TOURTELOTTE WW. New Diagnostic Criteria for Multiple Sclerosis: guidelines for research protocols. Annals of Neurology 1983;13(3):227-231.
- KURTZKE JF. Rating neurology impairment in multiple sclerosis: an expanded disability status scale (EDSS) . Neurol 1983;33:1444-1452.9 – Slater RJ, LaRocca NG, Scheinberg LC. Development and testing of a minimal record of disability in multiple sclerosis. Ann N Y Acad Sc. 1984;436:453-68.
- LOWIS GW. Ethnic Factor in Multiple Sclerosis: a review and critique of the epidemiological literature. Intern J Epid 1988;17:14-20.
- ALVARENGA RMP, LEON SVA, SANTOS CMM, ALVARENGA H, SOHLER MP. Multiple sclerosis in Brazil: characteristics according to ethnic influence In: XVII WORLD CONGRESS OF NEUROLOGY , 2001 , London. Journal of the Neurological Sciences 2001;187: S323 -S324.
- MIRANDA-SANTOS CM – PROTOMS: Protótipo computadorizado para avaliação da esclerose múltipla – Dissertação (Mestrado em Neurologia). Universidade Federal Fluminense. Niterói:(s.n.),2001.S237. Pag 30.