
|
|
|
CASE REPORT / CAS CLINIQUE
TRANSIENT MUTISM AFTER ANTERIOR TRANSCALLOSAL APPROACH
MUTISME TRANSITOIRE APRÈS UN ABORD ANTÉRIEUR TRANSCALLEUX
E-Mail Contact - NAAMA Okacha :
okacha_naama@hotmail.com
ABSTRACT Postoperative mutism is an infrequent complication of brain surgery. We report a third ventricular astrocytoma in a 16-year-old boy. The tumor was totally removed via anterior transcallosal approach. The operation was uneventful. On the second postoperative day he became mute. He could follow verbal commands, and write and read. Histopathological examination revealed a pilocystic astrocytoma. Four days postoperatively, he began to say simple words, and two weeks later he could talk normally. The possible cause and pathophysiological mechanism of mutism in the lesions of this region are discussed in this paper Key words: Mutism; Third ventricle; Corpus callosum; Transcallosal approach Mots clés: Abord trans calleux; Corps calleux; Troisième ventricule INTRODUCTION The term mutism is usually defined as a clinical state in which a conscious patient is unwilling to or unable to speak, resulting in the absence or marked paucity of verbal output. The patients may be mute while they are willing to write, or intact in comprehension (10). Mutism may be a result of division of the corpus callosum. Suppression of the limbic system caused by lesions in the anterior cingulate gyrus, septum pellucidum, and fournix may also have been of importance in at least two of these three cases. Impairments of the supplementary motor cortex, thalamus and basal ganglia may also contribute to reduce speech production (3). The mechanism of such transient mutism seems to be a complex of two or more of these factors, and their combination may be different from one case to the other (6). A few authors have contributed their case materials but the overall number of cases remains small. We present a patient who developed transient mutism after resection of a third ventricular astrocytoma. CASE REPORT A 16-year right-handed was referred for further management of a third ventricular lesion, which resulted in obstructive hydrocephalus that was treated with a right ventriculoperitoneal shunt. Three month before admission, the patient experienced increasing episodes of vomiting and gait disturbances. His family history was unremarkable for neurological diseases or phacomatosis syndromes. The general status of the patient was satisfactory. There was no papilledema or restriction in his extraocular movements. The cranial nerves were intact, and there were no sensory or motor deficits. The Romberg test was normal. The patient was able to heel and toe walk, but his tandem gait was clumsy. Deep tendon reflexes were symmetrical, and Babinski’s sign was absent. No stigmata of neurofibromatosis was noted. Computed tomographic and magnetic resonance imaging with contrast demonstrated a homogeneously enhancing mass measuring 3 × 2,5 × 3,5 cm in the third ventricle. The patient underwent a right frontoparietal craniotomy with an extension of the bone flap across the midline and two-thirds of the bone flap anterior to the coronal suture. The interhemispheric fissure was identified and retracted down to the edge of the falx. The left and right cingulate gyri were spread, and the corpus callosum was identified. A midline dissection, measuring 2 cm, was performed through the corpus callosum. After passing through the corpus callosum, the leaves of the septum pellucidum were separated, and the ependyma of the third ventricle was encountered. The ependyma was opened, and the tumor was visualized. The right lateral ventricle was then entered, and cerebrospinal fluid was removed to provide further relaxation. The tumor appeared grayish-white with a firm consistency. We were able to find a relatively clean capsule margin between the tumor and the third ventricle along the anterior, superior, and lateral portions of the dissection. A gross total resection could be achieved. Histopathologic evaluation confirmed the diagnosis of pilocytic astrocytoma. Operation and recovery from anesthesia were uneventful. In response to verbal commands the patient could open his mouth. On the second postoperative day, he was alert but totally mute. He suffered fits of crying. He could follow verbal commands and was able to read and write. A follow-up CT scan on the second postoperative day, showed normal postoperative changes with no haematoma or significant oedema. Four days postoperatively, he began to say simple words, and two weeks later he could talk normally. DISCUSSION Mutism caused by intracranial surgical intervention has been described in the following areas: (i) Broca’s area; (ii) the anterior cingulate area; (iii) the descending tracts bilaterally; (iv) the mesencephalic reticular formation; (v) after callosotomy; (vi) the SMA; and the midline cerebellar structures (5,10). Transient mutism is frequently observed following callosal operations, the nature of this disorder has remained elusive (6). Rayport (7) reported mutism in three patient following section of the posterior corpus callosum. Sass (8) reported that two of 12 patients who underwent complete callosotomy showed a period of mutism, whereas only one of 20 patients who underwent anterior callosotomy experienced similar transient mutism. Such results seem to suggest an important role of the splenium of the corpus callosum in precipitating the transient mutism, and the anterior part of the corpus callosum looks to be relatively innocent (3). In a series of three patients with transient mutism after anterior transcallosal approach, all of them underwent a callosal incision of 2-3 cm and removal of the septum pellucidum, suggesting that a small anterior callosal incision behind the genu is not responsible for mutism (6). By contrast, several other report indicate that the surgical section of the anterior body of the corpus callosum may well cause transient mutism (4). Shucart (9) reported that one of 25 cases operated on through an anterior transcallosal approach showed mutism. Geuna (4) reported that a patient with an intraventricular oligodendroglioma became transiently mute after an anterior trancallosal operation. Asgari (2) demonstrated that postoperative transient mutism was not limited to the anterior callosotomy but also occurred in the frontal transcortical approach, and all of these patients postoperative mutism was due to damage to the septal region in cases of tumor involvement of the septum pellucidum or damage to both fornices in cases of large solid third ventricular tumors rather than to the callosal incision itself. It is evident that the surgical section of the corpus callosum is not by itself sufficient to cause the transient mutism, because many subjects who underwent such an operation remain free of mutism after the procedure (3,4). Therefore the mechanism of such transient mutism seems to be a complex of two or more of these factors, and their combination may be different from one case to the other (6,10). More likely, this syndrome occurs in individuals with mixed cerebral dominance for language function. Sass (8) found that right-handed epileptic patients that are right-hemisphere dominant for speech develop permanent speech difficulties after callosotomy. It is possible that in some right-handed patients who develop left cerebral dysfunction, language function shifts incompletely to the right hemisphere. Speech and other language functions in these patients may depend on interhemispheric communication that is interrupted by a callosal incision. The factors which may contribute to the development of transient mutism following callosal surgery may be classified into two groups; one is an individual variation in organization of linguistic functions mediated by the corpus callosum, and the other is a second surgical lesion other than the one in the corpus callosum (6). Factors related to the section of the corpus callosum seem to include the following (3,6): (a) the commissural fibers may have been acting in a compensatory fashion because of previous brain injury. (b) speech may require some absolute number of relevant brain connections. (c) the callosotomy may produce a diaschisis in the speaking hemisphere, from which it recovers only slowly. (d) the callosum may carry a corollary discharge for speech output whose sudden loss results in downstream interhemispheric conflict. (e) temporary circulatory alterations (particularly of the internal cerebral veins) may transiently derange the more sensitive functions of the basal ganglia. (f) damage to one or the other fornix may be contributory. (g) trauma to the anterior third ventricle during division of the anterior commissure or during division of the rostrum from in front may be relevant. (h) supplementary motor cortex dysfunction may be contributory. (i) decussating fibers (from medial frontal cortex to opposite basal ganglia) may be relevant so that their interruption plus medial frontal cortex edema combines to produce mutism when associated with a thalamic or striatal lesion. The outcome for postoperative mutism is favorable (1). Slight disturbance of speech production may result from right-handed patient. But permanent, significant aphasia from such lesions is rare. In addition to deficits concerning initiation-related functions, several patients were unable to inhibit certain inappropriate speech and motor functions (10). In most reported cases postoperative mutism is described as a transient abnormality and it resolves in less than 2 weeks with no treatment (1), as in our case. In rare cases such as when dopaminergic pathways are injured, dopamine replacement may be necessary (10). CONCLUSION The major causes in the genesis of postoperative mutism would seem to be multi-factorial, including a disconnection of the callosal fibers and/or impairment of the related neural structures, such as the cingulum, and possibly others. FIGURES 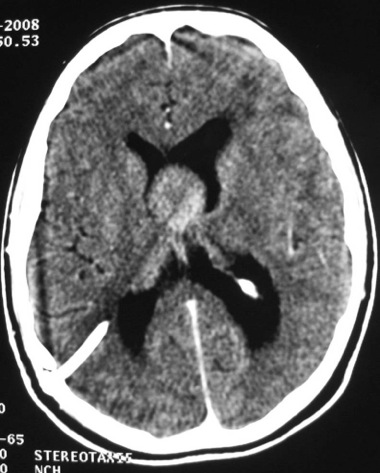 Figure 1a 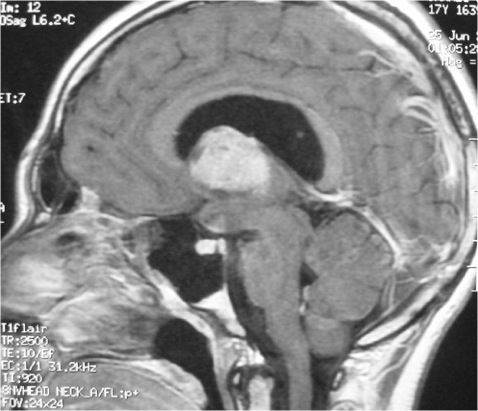 Figure 1b 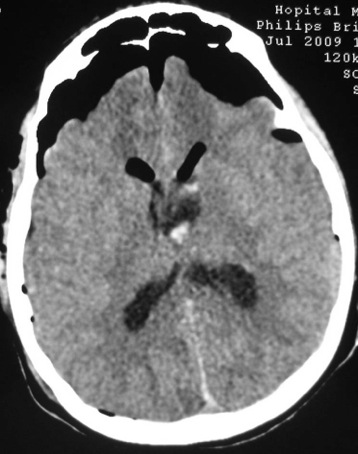 Figure 2 REFERENCES
|
© 2002-2018 African Journal of Neurological Sciences.
All rights reserved. Terms of use.
Tous droits réservés. Termes d'Utilisation.
ISSN: 1992-2647