
|
|||||||||||||
|
REVIEW / MISE AU POINT
HUNTINGTON’S DISEASE: A PERPLEXING NEUROLOGICAL DISEASE
E-Mail Contact - GUNDAMARAJU Rohit :
rohit.gundamaraju@gmail.com
ABSTRACT Huntington’s disease is an inherited intricate brain illness. It is a neurodegenerative, insidious disorder; the onset of the disease is very late to diagnose. It is caused by an expanded CAG repeat in the Huntingtin gene, which encodes an abnormally long polyglutamine repeat in the Huntingtin protein. Huntington’s disease has served as a model for the study of other more common neurodegenerative disorders, such as Alzheimer’s disease and Parkinson’s disease. Symptomatic treatment of Huntington’s disease involves use of Dopamine antagonists, presynaptic dopamine depleters, Antidepressants, Tranquillizers,Anxiolytic Benzodiazepines, Anticonvulsants and Antibiotics. Several medications including baclofen,idebenone and vitamin E, Zinc-finger nucleases (ZFNs) have been studied in clinical trials with limited samples. In the present article, we have concentrated on clinical features, diagnosis, symptomatic approaches, symptomatic treatment and other therapies involved in the management of Huntington’s disease. Keywords: Neurodegeneration, Polyglutamine, Huntingtin, Symptomatic treatment INTRODUCTION Huntington’s disease (HD) is a neurodegenerativegenetic disorder that affects muscle coordination and leads to cognitive decline and psychiatric problems. It typically becomes noticeable in mid-adult life. [1] HD is the most common genetic cause of abnormal involuntary writhing movements called chorea, which is why the disease used to be called Huntington’s chorea. [4]For many decades its name remained unchanged, until the nineteen-eighties when, fully aware of the extensive non-motor symptoms and signs, the name was changed to Huntington’s disease (HD). [2] In 1983, a linkage on chromosome 4 was established and in 1993 the gene for HD was found. For the first time, actual pre-manifest diagnoses could be made and as more diseases involving trinucleotide repeats of CAG were found, HD served as a model for many studies in medicine. CAG (cytosine (C), adenine (A), and guanine (G)), is a trinucleotide, the building stone of DNA. CAG is the codon for the amino acid glutamic. Finding the gene opened new research lines, new models and for the first time a real rationale on the way to treat this devastating disease. Many symptomatic treatments are now available, but there is a need for better, modifying drugs. [3] HD is named after George Huntington, the physician who described it as hereditary chorea in 1872. [3, 4]  Figure 1 EPIDEMIOLOGY HD affects males and females in relatively equal numbers. The disorder occurs in various geographic and ethnic populations worldwide. The frequency of HD appears to vary among different populations, ranging from an estimated 4 to 10 individuals in 10,000.[6]Huntington’s disease shows a stable prevalence in most populations of white people of about 5-7 affected individuals per 100000. [7] Exceptions can be seen in areas where the population can be traced back to a few founders, such as Tasmania and the area around Lake Maracaibo in Venezuela. In Japan, prevalence of the disorder is 0.5 per 100 000, about 10% of that recorded elsewhere, and the rate is much lower in most of Asia. African populations show a similarly reduced prevalence, although in areas where much intermarriage with white people takes place the frequency is higher. [6, 7] Currently, the higher incidence of Huntington’s disease in white populations compared with African or Asian people relates to the higher frequency of Huntingtin alleles with 28-35 CAG repeats in white individuals. [8] SIGNS AND SYMPTOMS Huntington’s disease is a widely variable disorder, even within the same family. The early symptoms of Huntington’s disease generally include slight, uncontrollable muscular movements, Chorea, stumbling and clumsiness, lack of concentration, lapses of short-term memory, depression, and changes of mood, sometimes including aggressive or antisocial behavior. [2, 3]The rate of progression of Huntington’s disease varies, but generally, it develops over 15-25 years. [5] Later in the illness, people may experience different symptoms, which include involuntary movements, Difficulty in speech and swallowing, Weight loss as well as emotional changes, resulting in: Stubbornness, Frustration, Mood swings, Depression. Chorea (derived from the Greek word meaning to dance) is the most common movement disorder seen in HD. [4] Chorea is a characteristic feature of HD and, until recently; the disorder commonly was called Huntington chorea. [9] Chorea, as defined by the World Federation of Neurology, is a state of excessive, spontaneous movements, irregularly timed, randomly distributed, and abrupt which is evidently illustrated in figure 2. Severity of chorea may vary from restlessness with mild intermittent exaggeration of gesture and expression, fidgeting movements of the hands, and unstable dance like gait to a continuous flow of disabling violent movements. Initially, mild chorea may pass for fidgetiness. [2] Severe chorea may appear as uncontrollable flailing of the extremities (i.e., ballism), which interferes with function.  Figure 2 Cognitive decline is characteristic of HD, but the rate of progression among individual patients can vary considerably. Dementia and the psychiatric features of HD are perhaps the earliest and most important indicators of functional impairment. [4]The dementia syndrome associated with HD includes early onset behavioral changes, such as irritability, untidiness, and loss of interest. PATHOLOGENOIUS The selective neuronal dysfunction and subsequent loss of neurons in the striatum, cerebral cortex, and other parts of the brain can explain the clinical picture seen in cases of HD. Several mechanisms of neuronal cell death have been proposed for HD, including excitotoxicity, oxidative stress, impaired energy metabolism, and apoptosis. [10] I. Exciting toxicity Excitotoxicity refers to the neurotoxin effect of excitatory amino acids in the presence of excessive activation of postsynaptic receptors. Intrastriatal injections of kainic acid, an agonist of a subtype of glutamate receptor, produce lesions similar to those seen in HD. [11] Intrastriatal injections of quinolinic acid, an N-methyl-D-aspartate (NMDA) receptor agonist, selectively affect medium-sized GABA-ergic spiny projection neurons, sparing the striatal interneuron’s and closely mimicking the neuropathology seen in HD. NMDA receptors are depleted in the striata of patients with HD, suggesting a role of NMDA receptor-mediated excitotoxicity, but no correlation exists between the distribution of neuronal loss and the density of such receptors.[5, 11] The theory that reduced uptake of glutamate by glial cells may play a role in the pathogenesis of HD also has been proposed. II. Oxidative stress Oxidative stress is caused by the presence of free radicals (i.e., highly reactive oxygen derivatives) in large amounts.[12] This may occur as a consequence of mitochondrial malfunction or excitotoxicity and can trigger apoptosis. Striatal damage induced by quinolinic acid can be ameliorated by the administration of spin-trap agents, which reduce oxidative stress, providing indirect evidence for the involvement of free radicals in excitotoxic cell death. [13] III. Impaired energy metabolism Impaired energy metabolism reduces the threshold for glutamate toxicity and can lead to activation of excitotoxic mechanisms as well as increased production of reactive oxygen species.[8] Nuclear magnetic resonance spectroscopy studies have shown elevated lactate levels in the basal ganglia and occipital cortex of patients with HD.[12] Patients with HD have an elevated lactate-pyruvate ratio in the cerebrospinal fluid. A reduction in the activity of the respiratory chain complex II and III (and less in complex IV) of mitochondria of caudate neurons in patients with HD has been reported. [14] 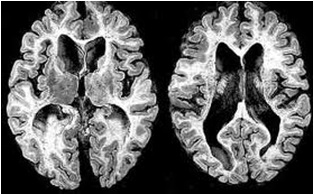 Figure 3 The genetic basis of HD is the expansion of a cysteine-adenosine-guanine (CAG) repeat encoding a polyglutamine tract in the N -terminus of the protein product called Huntingtin. The function of Huntington is not known. Normally, it is located in the cytoplasm. [19, 20] The association of Huntingtin with the cytoplasmic surface of a variety of organelles, including transport vesicles, synaptic vesicles, microtubules, and mitochondria, raises the possibility of the occurrence of normal cellular interactions that might be relevant to neuro-degeneration. N-terminal fragments of mutant Huntingtin accumulate and form inclusions in the cell nucleus in the brains of patients with HD, as well as in various animal and cell models of HD. [18] The presence of neuronal intra nuclear inclusions (NIIs) initially led to the view that they are toxic and, hence, pathogenic. More recent data from striatal neuronal cultures transfected with mutant Huntingtin and transgenic mice carrying the spinocerebellar at axia-1 (SCA-1) gene (another CAG repeat disorder) suggest that NIIs may not be necessary or sufficient to cause neuronal cell death, but translocation into the nucleus is sufficient to cause neuronal cell death.Caspase inhibition in clonal striatal cells showed no correlation between the reduction of aggregates in the cells and increased survival. [19] PATHOLOGY The most striking pathology in HD occurs within the neostriatum, in which gross atrophy of the caudate nucleus and putamen is accompanied by selective neuronal loss and astrogliosis. Marked neuronal loss also is seen in deep layers of the cerebral cortex. [16] Other regions, including the globuspallidus, thalamus, sub thalamic nucleus, substantianigra, and cerebellum, show varying degrees of atrophy depending on the pathologic grade. [17] The extent of gross striatal pathology, neuronal loss, and gliosis provides a basis for grading the severity of HD pathology. No gross striatal atrophy is observed in grades 0 and 1. Grade 0 cases have no detectable histologic neuropathology in the presence of a typical clinical picture and positive family history suggesting HD. [16, 17] Grade 1 case have neuropathological changes that can be detected microscopically but without gross atrophy. In grade 2, striatal atrophy is present, but the caudate nucleus remains convex. In grade 3, striatal atrophy is more severe, and the caudate nucleus is flat. In grade 4, striatal atrophy is most severe, and the medial surface of the caudate nucleus is concave. [17, 18] 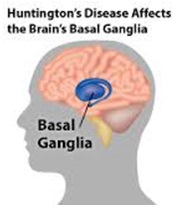 Figure 4 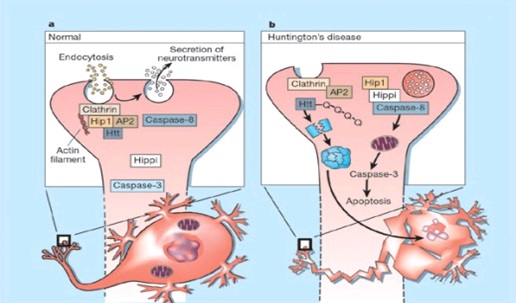 Figure 5 HUNTINGTIN The Huntington’s disease is still not fully understood. What is known about Huntington’s disease is that it occurs due to mutations in a single gene coding for a protein called Huntington. [20]Huntingtin is expressed in all human and mammalian cells, with the highest concentrations in the brain and testes; moderate amounts are present in the liver, heart, and lungs. [21] Recognizable autologous of protein is present in many species, including zebra fish, drosophila, and slime moulds. The role of the wild-type protein is, yet, poorly understood, as is the underlying pathogenesis of Huntington’s disease.[21, 22]One mechanism by which an autosomal-dominant disorder such as Huntington’s disease could cause illnesses by haploin sufficiency, in which the genetic defect leads to inadequate production of a protein need for vital cell function.This idea seems unlikely because terminal deletion or physical disruption of the HDgene in man does not cause Huntington’ disease. [15, 16] Furthermore, one copy of the HDgene does not cause a disease phenotype in mice. Whereas homozygous absence of the HDgene is associated with embryonic lethality in animals, people homozygous for the HDgene have typical development.Findings suggest that the mutant HDgene confers toxic gain of function. [3, 4, 5] A persuasive line of evidence for this idea comes from nine other known human genetic disorders with expanded (and expressed) polyglutaminerepeats: spinocerebellar ataxia types, Dentatorubropallidoluysian atrophy, and spinobulbar muscular atrophy. For none of these disorders is there evidence to suggest an important role for haplo insufficiency. [24] In spinobulbar muscular atrophy, complete deletion of the androgen receptor is not associated with neuromuscular disease. All nine diseases show neuronal inclusions containing aggregates of polyglutamines and all have a pattern of selective neuro-degeneration.One of the most striking features of these disorders is the robust inverse correlation between age of onset and number of polyglutamine repeats. [10] Results suggest that the length of the Polyglutamine repeat indicates disease severity irrespective of the gene affected, with the longest repeat lengths associated with the most disabling early-onset (juvenile) forms of these disorders. Although difficult to confirm, some data also suggest that the rate of progression might be faster with longer CAG repeats, particularly for individuals with juvenile-onset disease. [19]The most convincing evidence for a gain of function in Huntington’s disease is the structural biology of polyglutamine strands. [10, 11] This process needs a specific concentration of protein and a minimum of 37 consecutive glutamine residues, follows a period of variable abeyance and proceeds faster with higher numbers of glutamine repeats. These findings might account for both delayed onset of disease and the close correlation with polyglutamine length. The rate of aggregation increases with the number of glutamine residues, which accords with evidence showing that length of expansion is associated with early age of onset. [2, 3, 10] CLINICAL FINDINGS Individuals with Huntington’s disease can become symptomatic at any time between the ages of 1 and 80 years; before then, they are healthy and have no detectable clinical abnormalities. [1, 5]This healthy period merges imperceptibly with a pre-diagnostic phase, when patients show subtle changes of personality, cognition, and motor control. Both the healthy and pre-diagnostic stages are sometimes called presymptomatic, but in fact, the pre-diagnostic phase is associated with findings, even though patients can be unaware of them. Diagnosis takes place when findings become sufficiently developed and specific. In the pre-diagnostic phase, individuals might become irritable or disinherited and unreliable at work; multitasking becomes difficult and forgetfulness in addition, anxiety mounts.Family members note restlessness or fidgeting, sometimes keeping their partners awake at night. [8, 31] Eventually, this stage merges with the diagnostic phase, during which time affected individuals show distinct chorea, in coordination, motor impersistence, and slowed saccadic eye movements. Cognitive dysfunction in Huntington’s disease, often spares long-term memory but impairs executive functions, such as organizing, planning, checking, or adapting alternatives, and delays the acquisition of new motor skills. These features worsen over time; speech deteriorates faster than comprehension. [9]Unlike cognition, psychiatric and behavioral symptoms arise with some frequency but do not show stepwise progression with disease severity. Depression is typical and suicide is estimated to be about five to ten times that of the general population (about 5-10%). [4] Manic and psychotic symptoms can develop. Suicidal ideation is a frequent finding in patients with Huntington’s disease. In a cross-sectional study, about 9% of asymptomatic at-risk individuals contemplated suicide at least occasionally, perhaps a result of being raised by an affected parent and awareness of the disease. In the pre-diagnostic phase, the proportion rose to 22%, but in patients who had been recently diagnosed, suicidal ideation was lower. The frequency increased again in later stages of the illness. [6, 7] The correlation of suicidal ideation with suicide has not been studied in people with Huntington’s disease, but suicide attempts are not uncommon. In one study, researchers estimated that more than 25% of patients attempt suicide at some point in their illness. Individuals without children might be at amplified risk, and for these people access to suicidal means (i.e., drugs or weapons) should be restricted. The presence of affective symptoms, specific suicidal plans, or actions that increase isolation (e.g., divorce, giving away pets) warrants similar precautions.Although useful for diagnosis, chorea is a poor marker of disease severity. Patients with early onset Huntington’s disease might not develop chorea, or it might arise only transiently during their illness. [1, 5] JUVENILE HD If the first symptoms and signs start before the age of 20 years, the disease is called Juvenile Huntington’s disease (JHD). Behaviors disturbances and learning difficulties at school are often the first signs. Motor behavior is often hypokinetic and bradykinetic with dystonic components. Chorea is seldom seen in the first decade and only appears in the second decade. Epileptic fits are frequently seen. The CAG repeat length is over 55 in most cases. In 75% of the juveniles the father is the affected parent. [21, 19] A complete and accurate family history is invaluable in evaluating a child with symptoms suggestive of Huntington disease, and can make the process of diagnosis much more straightforward. [22] However, there are situations in which parents may not even be aware that HD is in the family; in other cases, an adopted child may be involved. A number of tests may be used in conjunction with the presence or absence of a family history, and clinical presentation, to help clarify the diagnosis. Inheritance 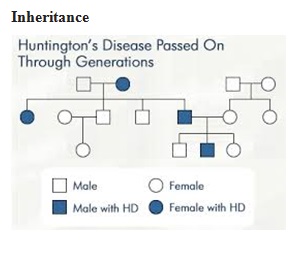 Figure 6 It is elucidated with example in the figure 6 that the dark colored animation is being affected and semi darks are at risk and finally the light colored are not affected. DIAGNOSIS Diagnosis of HD is not easy andrequires an extensive care while diagnosing. The discovery of the HD gene in 1993 resulted in a direct genetic test to make or confirm a diagnosis of HD in an individual who is exhibiting HD-like symptoms. Using a blood sample, the genetic test analyzes DNA for the HD mutation by counting the number of repeats in the HD gene region. [11, 15]Pre-symptomatic testing is used for people who have a family history of HD but have no symptoms themselves. The blood test confirms the presence or absence of the HD mutation. It is encouraged that patients have either a blood sample from a family member who has HD or the results of his/her genetic test for confirming the diagnosis. [31] If either parent had HD, the person’s chance would be 50-50.Prenatal testing is one of a range of options, which may be of interest to couples who are at risk of passing the disease-causing version of the HD gene to a child. And also, chorionic Villi Sampling is done to identify the presence of HD. In the past, no laboratory test could positively identify people carrying the HD gene or those fated to develop HD before the onset of symptoms. [25] 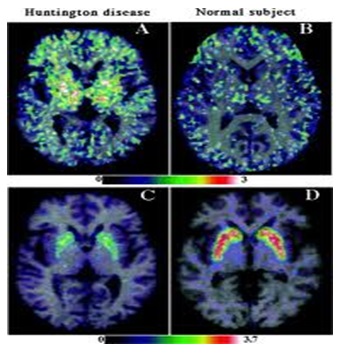 Figure 7 Upon presence of contrast agent, the microglial cells in HD and normal brain are well assessed. Imaging technologies allow investigators to view changes in the volume and structures of the brain and to pinpoint when these changes occur in HD. Scientists know that in brains affected by HD, the basal ganglia, cortex, and ventricles all show atrophy or other alterations. [20] TREATMENT There is no cure for Huntington’s disease, and there is no known way to stop the disease from getting worse. The goal of treatment is to slow down the symptoms and help the person function for as long and as comfortable as possible. [26, 30]
Table 1 : Symptomatictreatmentregime [26-28, 30] Table 1 is an overview of treatment options for HD. As there is no complete cure, a symptomatic treatment regime is followed. In August 2008, the U.S. Food and Drug Administration approved Tetrabenazine to treat chorea, making it the first drug approved for use in the United States to treat the disease. [28] In the year 2012, a clinical study is initiated on Zinc-finger nucleases (ZFNs) for treating HD. Zinc-finger nucleases (ZFNs) are artificial restriction enzymesgenerated by fusing a zinc finger DNA-binding domain to a DNA-cleavage domain. [33] Expanded CAG/CTG repeat tracts are the genetic basis for more than a dozen inherited neurological disorders including Huntington’s disease, myotonic dystrophy, and several spinocerebellar ataxias. [20] It has been demonstrated in human cells that ZFNs can direct double-strand breaks (DSBs) to CAG repeats and shrink the repeat from long pathological lengths to short, less toxic lengths. CONCLUSION Several medications including baclofen, idebenone and vitamin E have been studied in clinical trials with limited samples. More research work and clinical trials should be done to search effective drug molecules that would provide a more rational approach in the treatment of Huntington’s disease. The more research should be aimed to develop therapeutic agents that alter the course of neurodegenerative disease like preventing neuronal death or stimulating neuronal recovery. As there is no specific cure for HD, the goal of current research is to develop treatments that can prevent, retard or reverse neuronal cell death. More specific treatments for Huntington’s disease should become feasible. REFERENCES
|
© 2002-2018 African Journal of Neurological Sciences.
All rights reserved. Terms of use.
Tous droits réservés. Termes d'Utilisation.
ISSN: 1992-2647