
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
CLINICAL STUDIES / ETUDES CLINIQUES
ACCURACY OF THE SIRIRAJ STROKE SCORE IN DIFFERENTIATING CEREBRAL HAEMORRAGHE AND INFARCTION IN AFRICAN NIGERIANS
E-Mail Contact - OGUN Shamsideen Abayomi :
yomiogun@skannet.com
ABSTRACT Background Objectif Methodology Results Conclusion Keywords: Africa, cerebral haemorrhage, cerebral infarction, Nigeria, stroke, CT-scan RESUME Introduction Objective Méthodologie Résultats Conclusion Mots clés : Accident vasculaire cérébral hemorragique, Accident vasculaire cérébral ischémique, A frique , Nigeria, tomodensitométrie INTRODUCTION Stroke is a common neurological problem accounting for a third of all deaths in western countries [25] and about 4.5% to 17% of all deaths [2,14] and 2.3% to 8.7% of all admissions [14,16] in hospital based studies in Nigeria. The majority of acute stroke is caused by cerebral infarction [12,16], although, there was a suggestion of an increase in the proportion of haemorrhagic stroke in our population [15]. Accurate and prompt clinical diagnosis is crucial in patients presenting with sudden onset focal neurological deficits. Hence it is important for clinicians to be able to distinguish between cerebral haemorrhage (CH) and cerebral infarction (CI) in cases of acute stroke, since clinical management of the two disorders differs substantially [5]. The diagnosis of stroke is largely clinical in most developing countries as very few centres have facilities for brain imaging. The clinical accuracy of distinction of stroke from non-stroke has a sensitivity of up to 95% (5,18] and specificity between 66 to 97% (9,10). However, this accuracy drops significantly when stroke subtypes have to be distinguished, with sensitivity of 68% and specificity of 67% [4,11,23]. Despite its limitations, Computerised Tomography (CT) scan has greatly improved the accuracy and precision of the diagnosis of stroke and its subtypes [9,14]. It has been recommended for all patients with clinical features of stroke because all subsequent therapeutic decisions depend on its result [1,23]. However, CTis not readily available in most centres in sub-Saharan Africa, where distance and cost limit access. Hence, most patients with stroke are treated without the benefit of a CT scan and they risk being inappropriately treated. Scoring systems based on discriminant analysis technique have been developed, such as the Guy’s hospital score [3] (also known as the Allen score) and the Siriraj Stroke Score (SSS) [17]. Clinical scores were also designed by multivariate logistic regression (6). These are simple, cheap and practical means of distinguishing CH from CI but are not sufficiently sensitive to replace CT scan. The scores were each developed on one group of patients in a single location and therefore need to be validated in as many other patient groups as possible. The Guy’s hospital score has been evaluated with data from the Oxfordshire community stroke project (OCSP) [10] and at the National Hospital for Nervous Diseases, London [19]. The Siriraj stroke score has been evaluated [17] in Siriraj hospital, Bangkok, Thailand and also at the Western infirmary, Glasgow [24]. The clinical score by Besson was also validated in the University Hospital of Grenoble, France [6]. None of them has been evaluated in an African population to the best of our knowledge. This study aimed to determine the sensitivity, specificity and accuracy of Siriraj scoring system in distinguishing between CH and CI in stroke patients confirmed by CT scan. METHODOLOGY Records of computerised tomography (CT) of the brain done between 1991 – 1999 at University College Hospital (UCH), Ibadan, and RADMED diagnostic centre, Lagos were reviewed. The CT brain scans of all patients referred with clinical diagnosis of stroke were retrieved and reviewed by two of the authors (A.O.,B.F). The case notes of these patients at the referral hospitals were also retrieved and reviewed by the neurologists (S.O.,O.O.,F.O) in the team. A questionnaire was designed to extract relevant clinical data from the case records. The questionnaire recorded the age, sex, date of admission and discharge from hospital, presence of headache, vomiting, loss of consciousness, the level of blood pressure, history of hypertension, transient ischaemic attacks, diabetes mellitus, obesity, angina pectoris, intermittent claudication, haemoglobinopathy, atrial fibrillation and cholesterol level. The stroke type and anatomic localisation were also recorded. Some patients were comatose on admission and the history was obtained from relations. Only patients with adequate clinical notes were included in the study. The Siriraj stroke score was calculated as (2.5 x level of consciousness) + (2 x vomiting) + (2 x headache) + (0.1 x diastolic blood pressure) – (3 x atheroma markers) – 12. A score above +1 indicates intracerebral haemorrhage, while a score below -1 indicates infarction. A score between -1 and +1 represents an equivocal result needing a CT scan to verify the diagnosis. (Appendix 1) The SSS was computed for each patient, and based on the individual score, the patients were classified into CH or CI using the criteria > +1 for CH and < -1 for CI [17]. Patients with scores between +1 and -1 were unclassified. The classification of the stroke subtypes using SSS was compared with the CTscan diagnosis, which was taken as the gold standard. The CT of the brain was done with G.E. CT. MAX 640 in Lagos and G.E. 9000 in Ibadan, using 5mm slices at 5mm intervals at the base of the skull and 10mm slices at 10mm intervals for the rest of the brain. Contrast enhancement was performed only in patients with atypical hypodense lesions. The interval between the time of the culpable ictus and the CT scan was recorded and ranged from 5 to 15 days with a mean of 10 ± 0.6 days. Data analysis for sensitivity, specificity, accuracy, frequency distribution, histogram, receiver-operating characteristic curve and cut-off determination of the scores were performed with the Epi-info software and by standard statistical methods [20]. RESULTS The brain CT scans of 182 patients referred with clinical diagnosis of stroke were reviewed, 93 from University College Hospital (UCH) and 89 from Radmed. Of these, only 96 patients (53%) had complete clinical records and CT scan features consistent with the diagnosis of stroke, and these formed the subjects of this study. Of the remaining 86 patients, 59 (32%) had non-stroke lesions and 21 (12%) had incomplete clinical records. The case notes of six (3%) of the patients could not be retrieved at the referral centre. Of the 96 patients with stroke and complete clinical records, 67 were males and 29 were females. The male: female ratio was 2.4:1. The age of the patients ranged from 51 to 69 years with a mean of 60 ± 4.3 years. Eleven patients (12%) were comatose on presentation and history from the relations was relied upon. Fifty-two patients (54.2%) had CT scan features of CI, whilst 44 patients (45.8%) had features consistent with the diagnosis of CH. Using the Siriraj Stroke Score, 88 patients (91.7%) were classified, 48 (50%) as CI and 40 (41.7%) as CH. Eight patients (8.3%) had indeterminate scores. The frequency distribution of the scores of the patients grouped according to CTdiagnosis is shown in figure 1. The scores for patients with CTconfirmed CH ranged from -3.0 to +10.0, with a mode of +3.0, median of +0.5 and a mean of + 0.8 ± 2.3. For CTconfirmed CI, the range of the scores was from -6.0 to +4.0, with a mode of -3.0, median of -1.0 and a mean of -0.9 ± 2.6. Table II shows the frequency distribution, sensitivity, specificity and accuracy of each score for patients with Ctdiagnosis of CH, whilst Table III shows those for patients with CI. The best separation or highest accuracy was 63.6% for CH and 59.6% for CI and these occurred at scores of +3 and -3 respectively. At this cut-off, the specificity was 90.9% and 82.7% for CH and CI respectively but the corresponding sensitivity dropped to 36.0% and 36.4% respectively. However, at this cut-off scores, 25 (27.6%) of the 96 patients could not be classified compared to eight (8.3%) with the standard cut-off of -1 and +1. Thus, no optimal sensitivity, specificity and accuracy were achieved in this study. The Receiver-operating characteristic curve also showed no optimal cut-off (figure 2). DISCUSSION The Siriraj Stroke Score (SSS) had a sensitivity of 50% for cerebral haemorrhage (CH), and 58% for cerebral infarction (CI), with accuracy of 54.2% in this study. This is lower than the sensitivity of 89% for CH and 93% for CI, with accuracy of 90% reported in Bangkok, Thailand [17].It is possible that the SSS may not be sufficiently sensitive to differentiate between CH and CI in non-Asian population since our findings are comparable to the study by Weir and his co-workers [24], who showed an overall accuracy of 64% for the SSS. They had suggested its limited use in differentiating between CH and CI in populations other than those in Asia, and our findings appear to confirm this. The predictive value of any diagnostic score depends greatly on the prevalence of the disease in the area of study, and scoring systems may not be applicable transculturally [17]. In Thailand, there is a preponderance of haemorrhagic stroke while in the Nigerian stroke population cerebral infarction is commoner [16]. Cerebral haemorrhage accounts for 40 – 50% of strokes in Thailand [22], 19% in Nigeria [16], 10 – 15% in Europe [6], and 20% in America [7]. Siriraj Stroke Score appears to have high predictive values in populations with preponderance of haemorrhagic stroke as in Asia, and low predictive values in populations with preponderance of cerebral infarction as in Africa, Europe and America. We were surprised that the predictive values amongst Nigerians were low despite the recent suggestion of an increasing proportion of haemorrhagic stroke in our population [15]. Our findings do not support the view of Celami et al. that the use of SSS is probably better in detecting infarction than haemorrhage, and supposedly should be applicable to Africans [8]. In this study, SSS was not sufficiently sensitive to differentiate between CI and CH using the acceptable discriminant clinical variables and the cut-off values. The questionable validity of accurate history in a retrospective study could be contributory, as 12% of the patients were comatose on presentation and reliance was placed upon history from relatives. History of headache at the time of ictus, past history of intermittent claudication or angina pectoris may not be obtained in such circumstances. The low occurrence of atheroma markers such as intermittent claudication or angina pectoris in this population could be additional factors [16]. Furthermore, the discriminant value of headache in distinguishing between haemorrhage and infarction was low in this study (table IV) in contrast to the findings of Besson et al. [6]. However, loss of consciousness and diastolic blood pressure above 100, had high discriminant value in accordance with their findings. The best separation or highest accuracy in this study was 63.6% for CH and 59.6% for CI at scores of +3 and -3 respectively. Our study showed that with increasing accuracy and specificity, the diagnostic sensitivity dropped significantly and as many as 27.6% of the patients were unclassified at the cutoffs that gave the best separation accuracy. Although, this proportion is comparable to the proportion of unclassified patients in the original validation study in which 20% of their patients were unclassified [17]. The Allen score was validated and found to have an overall predictive accuracy of 78% in Oxford [10] and 82% in London [19]. This was however not used for this retrospective study because it requires several historical and clinical details, and cannot be used until 24 hours after the stroke. Also, the clinical scores by Besson [6] had an empirical positive predictive value approaching 100%, but involve more clinical variables than the SSS. The SSS is easier to determine and can be used immediately after stroke. The overall predictive accuracy (64%) of Allen scores and the SSS was similar amongst Caucasians [3], therefore, the choice of either should depend on ease of use or other considerations. The SSS was chosen because it involves fewer variables with the greater possibility of having more complete data to calculate the score in this retrospective study. Moreover, it would be easier to apply in a busy, less well-equipped clinical setting, as is prevalent in developing countries of Africa.Although, this study has not supported the use of the SSS in our stroke patients using the discriminant clinical variables and the cut-off, it is unlikely that CT scan would be widely available and easily accessible to all stroke patients in sub-Saharan Africa in the near future. Therefore, the search must continue for a simple clinical scoring system to differentiate between CH and CI. A prospective study with larger sample size is suggested. Such a study may be able to determine a more accurate cut-off point. Modification of the discriminant clinical variables to exclude variables with low discriminant value and include variables with higher discriminant value in the African population should be considered. Until result of such a study is available, we contend that CTscan should remain the only reliable investigation for distinguishing between CH and CI among African Nigerians and it should be made available and affordable. TABLE 1 : SSS CLASSIFICATION INTO STROKE SUBTYPES IN PATIENTS WITH CT CONFIRMED DIAGNOSIS OF STROKE CT DIAGNOSIS
sensitivity for cerebral haemorrhage (CH) = 50% TABLE 2 : SENSITIVITY, SPECIFICITY, ACCURACY AND FREQUENCY DISTRIBUTION OF SCORES IN PATIENTS WITH CT DIAGNOSIS OF CEREBRAL HAEMORRHAGE
TABLE 3 : SENSITIVITY, SPECIFICITY, ACCURACY AND FREQUENCY DISTRIBUTION OF SCORES IN PATIENTS WITH CT DIAGNOSIS OF CEREBRAL INFARCTION
TABLE 4 : DISCRIMINANT VALUE OF CLINICAL VARIABLES IN THE CT SCAN CONFIRMED CEREBRAL HAEMORRHAGE AND INFARCTION
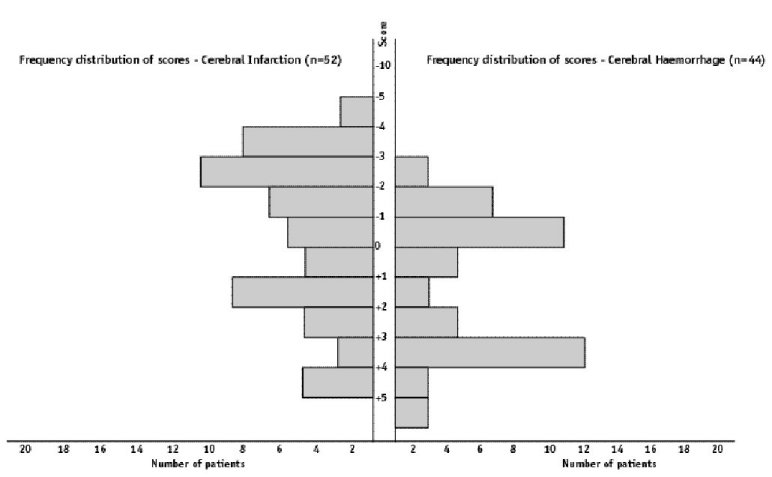 Figure 1: Histogram of the frequency distribution of scores for patients with CT diagnosis of cerebral infarction and cerebral haemorrhage 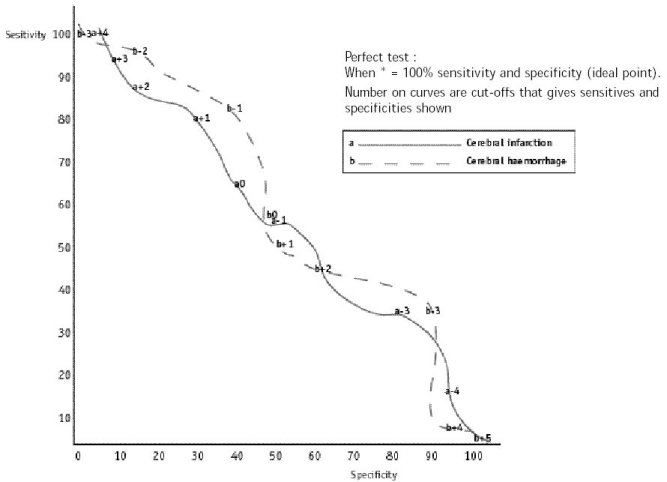 Figure 2 : receiver – operating characteristic curve REFERENCES
|
© 2002-2018 African Journal of Neurological Sciences.
All rights reserved. Terms of use.
Tous droits réservés. Termes d'Utilisation.
ISSN: 1992-2647