ORIGINAL PAPERS / ARTICLES ORIGINAUX
NEURO-OPHTHALMOLOGIC MANIFESTATIONS OF HUMAN AFRICAN TRYPANOSOMIASIS
MANIFESTATIONS NEURO-OPHTHALMOLOGIQUES DE LA TRYPANOSOMIASE HUMAINE AFRICAINE
- Centre for International Health, University of Bergen Bergen, Norway
- Department of Neurology, Kinshasa University Hospital Kinshasa Dem. Rep. of Congo
- Department of Neuro-ophthalmology, Moorfields Eye Hospital and The National Hospital for Neurology and Neurosurgery London, UK
ABSTRACT
Background
Human African trypanosomiasis is a re-emerging parasitic disease that affects the central nervous system and leads to severe neurologic manifestations in the late stage of the disease. Little is known about the prevalence of neuro-ophthalmologic disturbances in patients with human African trypanosomiasis (HAT).
Objectives
To determine the prevalence and the features of neuro-ophthalmologic abnormalities including the visual evoked potentials (VEPs) in HAT patients at the meningo-encephalitic stage prior to treatment.
Methods
Neuro-ophthalmologic examination was performed in 114 patients with neurologic involvement of HAT, of whom 110 underwent pattern reversal VEP recording.
Results
Neuro-ophthalmologic manifestations were observed in 32 patients (28%). Papilloedema (17%) and ocular motor nerve palsies (6%) mainly affecting the sixth cranial nerve were the most frequent neuro-ophthalmologic disorders. Visual field defects, cortical blindness and nystagmus were found in five, two and one patient respectively. In patients with cranial nerve palsies, the proportion of patients with papilledema was significantly greater than that of those without papilledema. A similar difference was observed between the proportion of patients with and that of those without parasite in CSF. VEPs were abnormal in 23% of the patients. The VEP abnormality consisted only of isolated P100 latency prolongation while the amplitude remained normal in all patients. VEPs were more likely to be abnormal in patients with papilloedema. There was no association between the prolongation of P100 latency and CSF biological parameters.
Conclusion
The most frequent neuro-ophthalmologic abnormalities in neurologically affected patients with HAT were those associated with increased intracranial pressure: papilloedema followed by cranial nerve palsies, mainly involving the abducens nerve. A few patients had pathological VEP despite apparently normal clinical examination. VEP is therefore a useful tool to detect subclinical impairment of the visual pathways in patients with HAT meningo-encephalitis.
Keywords : neuro-ophthalmologic manifestations, visual evoked potentials, human African trypanosomiasis
RESUME
Introduction
La trypanosomiase humaine africaine est une affection parasitaire qui affecte le système nerveux central et qui engendre des troubles neurologiques sévères au stade méningo-encéphalitique. Il n’existe cependant une rareté des données sur les manifestations neuro-ophthalmologiques de cette affection.
Objectifs
Déterminer la prévalence et décrire les caractéristiques des manifestations neuro-ophtalmologiques et des potentiels évoqués visuels chez les patients atteints de méningo-encéphalite à T. gambiense avant le traitement.
Methods
Un examen neuro-ophthalmologique a été réalisé chez 114 patients atteints de méningo-encéphalite à T.gambiense, parmi lesquels 110 ont fait l’objet d’un enregistrement des potentiels évoqués visuels.
Résultats
Les manifestations neuro-ophthalmologiques ont été observées chez 32 patients (28%). L’oedème papillaire (17%) et les paralysies des nerfs oculo-moteurs (6%) touchant principalement le nerf oculo-moteur externe étaient les manifestations neuro-ophtalmologiques les plus fréquentes. L’amputation du champs visuel, la cécité corticale et le nystagmus étaient observés respectivement chez 5, 2 et 1 patient. Dans le groupe des patients ayant des paralysies des nerfs crâniens, ceux avec papilloedème était significativement plus nombreux que ceux sans papilloedeme; la meme observation était faite en ce qui concerne la présence ou l’absence du parasite dans le LCR. Les potentiels évoqués visuels étaient pathologiques chez 23% des patients. Chez tous ces patients la latence était prolongée alors que l’amplitude était conservée. Le risque d’avoir un potentiel évoqué visuel pathologique était plus élevé chez les patients avec oedème papillaire. Il n’y avait aucune association entre la prolongation de la latence et les paramètres biologiques du LCR.
Conclusion
Les manifestations neuro-ophthalmologiques les plus fréquentes chez les patients atteints de méningo-encéphalite à T. gambiense sont celles associées à l’hypertension intracrânienne: oedème papillaire suive des paralysies oculo-motrices. Une petite proportion de patients ont des potentiels évoqués visuels pathologiques en dépit d’un examen clinique apparemment normal. Les potentiels évoqués visuels sont une bonne méthode pour détecter les atteintes subcliniques des voies visuelles chez les patients atteints de méningo-encéphalite à T. gambiense.
Mots clés: manifestations neuro-ophtalmologiques, potentiels évoqués visuels, trypanosomiase humaine africaine
INTRODUCTION
Human African Trypanosomiasis (HAT), also known as sleeping sickness, is a parasitic disease caused by the T. brucei complex. It is an important public health problem in sub-Saharan Africa where it is associated with a high degree of suffering and mortality. In the West and Central Africa, it is caused by Trypanosoma brucei gambiense transmitted to humans through the bite of the tsetse fly (Glossina species). According to recent estimations 60 million people from 36 sub-Saharan countries are at risk of contracting the disease, while 300,000-500,000 people are already infested. The Democratic Republic of Congo is one of the most affected countries, accounting for 20% of the total population at risk in Africa. A combination of poor surveillance and no vector control has resulted in the re-emergence of the disease now reaching a very high prevalence (6, 11, 17, 19). It is believed that the population movement from and to the affected areas due to the ongoing unstable situation in the country contributes to the spread of the disease. Kinshasa, the capital, until recently considered to be free from the infection, is currently showing an alarming picture because of an increasing number of cases within the city (i.e. 433 and 912 cases reported in 1998 and 1999 respectively) (2). Clinically, the advanced stage of the disease, which has a chronic course, manifests by severe neurologic disorders after the parasite has invaded the central nervous system (CNS). HAT may induce different neuro-clinical pictures including pyramidal or extrapyramidal, brain stem, bulbar or pseudo-bulbar, superficial or profound sensitive, hypothalamic syndromes and intracranial hypertension.
Extensive studies have been conducted on HAT, but they mostly focussed on basic research. This may explain why apart from reports on isolate cases (3, 13, 20), data on neuro-ophthalmologic investigations in HAT are not available. Moreover the need for more clinical research in HAT has been recently emphasised (16).
The overall aim of the present study was to determine the prevalence and the features of both the neuro-ophthalmological manifestations and visual evoked potentials in neurologically affected patients with HAT. In addition, the study aimed at relating the clinical and VEP abnormalities to the biological parameters.
PATIENTS & METHODS
PATIENTS
Patients were recruited among those patients routinely detected by mobile trypanosomiasis teams in Kinshasa and surrounding rural areas and referred for further investigations to the trypanosomiasis ward at the Department of Neurology, Kinshasa University Hospital, which is the central point for all Trypanosomiasis treatment in the capital. Because of the potentially toxic and lethal effects of melarsoprol used to treat the second stage of HAT, referred patients routinely undergo a pre-treatment check-up during the first week of admission with the aim to ascertain the diagnosis and stage the disease. During this pre-treatment week the diagnosis of HAT is reconfirmed based on the identification of the parasite in blood, lymph nodes and /or the cerebro-spinal fluid (CSF). Involvement of the CNS (stage 2) is considered when at least one of the following criteria is met: 1) isolation of the parasite in the CSF, 2) the combination of a) presence of the parasite in blood or lymph node aspirate plus b) biochemical alterations of the CSF consisting of an increased leucocyte count (over 5 cells/mm3) and an increase in CSF protein (above 45 mg %) and c) an abnormal neurological examination, which may consist of isolated or combined signs of meningitis, frontal, pyramidal, extrapyramidal or cerebellar involvement. Other causes of infectious meningo-encephalitis notably malaria, tuberculosis, cryptococcosis, toxoplasmosis and HIV-infection are also routinely excluded before diagnosis of the second stage of HAT. CSF pressure was not established quantitatively.
This study included all newly detected and untreated patients with second stage of HAT during two periods: July-October 2001 and August-December 2002, a total of 114 patients. Their age ranged from 4 to 71 years (mean: 32 ± 14 years). Sixty-two (54%) of them were men. In 61 patients (54%) the parasite was found in the CSF.
METHODS
Neuro-ophthalmological examination
All patients with second stage of HAT underwent neuro-ophthalmological examination and VEP recording prior to any treatment. Visual acuity testing was performed with Snellen charts. The pupils were checked for size, shape and light responses, and upper lids checked for ptosis and retraction. Ocular motility and deviation, including ductions and versions in all cardinal positions of gaze, and the testing of convergence ability were also evaluated. Strabismus was assessed by cover-uncover and alternate-cover tests. Direct ophthalmoscopy with special attention on optic disc border and color, macula and foveal reflexes to exclude any apparent retinal disease that could interfere with the VEP results. Visual fields were quantified by Goldmann perimetry. Examination of the anterior segment of the eye by slit-lamp (Haag-Streit 900) biomicroscopy was also done.
VEP recording procedure
VEPs were recorded in the patients and controls. The controls consisted of 76 healthy subjects (36 males and 40 females) with a mean age of 25 ± 15 years (range: 8-58) recruited among relatives of the patients, medical students and medical staff. The preparations for the VEP recording were performed in a darkened room illuminated by a dim red lamp. The active electrode was placed in the midline just above the inion, the reference electrode placed on the midline 2 cm anteriorly, and the forehead used as ground. The VEPs were elicited monocularly with an alternating checkerboard pattern of black and white rectangles on a TV monitor (Philips, Italy) placed at a distance of 1 meter in front of the subject. Each check measured 1.71 degrees of visual angle horizontally and 0.85 degrees vertically. The luminances of the black and white checks were 2 and 90 cd/m2 respectively, with a contrast of 90%. The patients were asked to fixate constantly a central target placed on the TV screen. The recording was performed using a 2-channel equipment (Cadwell 5200A, USA). One hundred stimulus responses were averaged using the manufacturer’s setting scale of 4. Amplifier parameters were automatically set to a sensitivity of 20 µV/div, sweep speed at 20 msec/div and the repetition rate at 2.11/sec. Filters were set at 1 Hz low cut and 70 Hz high cut. The latency of the P100 component and the peak-to-peak amplitude of N75/P100 were measured for each eye. In addition the P100 latency and amplitude interocular differences were calculated. These values were compared by considering the control mean ± 2.5 SD as the upper and lower limits of normal values of each of the above parameters. All values not within the corresponding limits were considered as abnormal. P values less than 0.05 were considered statistically significant.
RESULTS
We examined 114 HAT patients with neurologic involvement. The clinical examination diagnosed neuro-ophthalmologic disturbances in 32 patients (28%), Table 1. Neurological manifestations were grouped in five clusters: meningeal manifestations (i.e. headaches, stiff neck, Kernig and Brudzinski’s signs), extrapyramidal manifestations (muscular rigidity, tremor, akathisia, dystonia, akinesia, dyskinesia), pyramidal manifestations (i.e. exaggerated tendon reflexes, Babinski’s reaction, Mingazzini or Baré’s reactions), cerebellar manifestations (i.e. hypotonia, dysdiadochokinesia, intention tremor, past-pointing) and frontal manifestations (i.e. grasp, snout, root, and suck reflexes, change in personality consisting of inappropriate jocularity, loss of initiative and concern, akinetic mutism, disinhibition and general retardation) were diagnosed in these patients. Sixteen of them (50%) had manifestations from all five clusters, 12 (37%) had all but the manifestations from the pyramidal cluster and 4 (13%) had manifestations from three different clusters.
Papilloedema was the most common neuro-ophthalmologic disorder observed in 19 patients (17%). It was bilateral in all cases and only observed in those with severe involvement of the CNS based on the clinical picture. In 15 of them, it was the only neuro-ophthalmologic disorder. Cranial nerve palsies were present in 10 patients (9%) of whom 7 (6%) had unilateral ocular motor nerve palsies and 5 (4%) had facial nerve palsy. In the group of patients with cranial nerve palsies, there was a significant difference between the number of those with (n = 7) and without (n = 3) papilledema (p<0.05). Similarly, the number of those with (n = 9) was significantly greater than that of those without (n = 1) parasite in the CSF (p<0.05). Multiple ocular motor nerve palsies were present in a single case. Visual field defects, mostly consisting in concentric constriction, were observed in 4 patients (4%) though one had an enlarged blind spot in addition (Figure 1). Cortical blindness and a primary position horizontal jerk nystagmus were discovered in 2 and 1 patient, respectively.
VEPs were recorded in 110 patients, Table 2. VEP was abnormal in 25 patients (23%). The P100 latency was bilaterally prolonged in 23 patients while the N75/P100 amplitude remained within the 95% confidence interval in all patients. However taken as a group, the mean P100 latency (102.8 ± 9.6 ms) was not significantly different as compared to the mean value of the controls (100.8 ± 5.2 ms) (p>0.05, C.I: -0.3-4.4). No significant difference was observed in the mean amplitude of the two groups of subjects (p =0.062). Among the 25 patients with abnormal VEPs, 9 (36%) had papilloedema in addition while the remaining had no papilledema. Sixteen subjects had prolonged VEP latency despite apparently normal optic discs. There were no association between P100 latency and CSF inflammatory parameters (lymphocyte count: p = 0.4 and protein level: p = 0.7), Figure 2. Patients with papilloedema had a significantly prolonged P100 latency (112.3 ± 6.6 ms) compared to those without papilloedema (100.7 ± 8.9 ms) (C.I.: 7.2-15.8) (Figure 3), and had four times the risk for having prolonged P100 latency than those without papilloedema (odds ratio = 4.2). In contrast, no significant difference was found between the mean P100 latency patients with (102.4 ± 9.9 ms) and those without (103.1 ± 9.2 ms) parasite in the CSF.
DISCUSSION
We here report the first systematic study of neuro-ophthalmologic manifestations in neurologically affected patients with HAT prior to treatment. In this series, 32 patients out of 114 (28 %) manifested with neuro-ophthalmologic abnormalities.
Papilloedema (17%) was the most common abnormality found in this series. Apart from the study by Dutertre (5) in France where papilloedema was found in 3 out of 19 (16%) patients coming from known endemic areas, most existing data on papilloedema in HAT are confined to isolated case reports. What is the pathogenic mechanism of papilloedema in HAT? In infectious meningo-encephalitis, papilloedema generally results either from optic neuritis following the direct effect of the infectious agent on the optic nerve or from raised intracranial pressure. The optic neuritis mechanism is questionable as no patients had symptoms or signs consistent with the diagnosis of optic neuritis in this series. The normality of the visual acuity and visual field observed in most patients also speaks against this mechanism. Moreover, although more recent experimental studies have demonstrated that trypanosomes cross the blood-brain barrier (7, 12), the presence of the parasite in the human brain parenchyma has not been proven (10). Since increased intracranial pressure is a well-known feature of the second stage of the disease, it is much more plausible that the papilloedema arises from raised intracranial pressure resulting from cerebral edema after the parasite has diffusely invaded CNS (4, 9).
The resulting rise of intracranial pressure is also likely to be the underlying cause of the observed cranial nerve palsies. Indeed, of the 10 patients with cranial nerve palsies 7 had papilledema. Since in HAT many structures of the brain are involved, it is also possible that some of the resulting lesions play a role in the occurrence of cranial nerve palsies, which are less commonly seen due to raised intracranial pressure alone, such as the third and sixth palsies. We therefore conclude that most of the neuro-ophthalmological pathologies seen are caused by raised intracranial pressure.
Although papilloedema was found to be a risk factor for delayed P100 latency, it was also observed in the present series that VEP were prolonged in some patients without papilloedema. The effects of high intracranial pressure on visual function have been studied by others investigators, in particular in benign intracranial hypertension. Prolonged latencies have been reported in 17-55% of cases (8, 14, 15, 18) and may precede disturbances of visual acuity and visual fields (15). This finding tends to suggest the presence of subclinical dysfunction in the visual pathways of patients with HAT. However the underlying mechanism has to be clarified, as is the case for some of the cranial neuropathies in HAT and other dysfunctions in the nervous system of the affected individuals. In HAT, demyelination of the brain has been reported in late stages both in vivo and in vitro (1, 5). However none of the studies has focused on the issue as to whether or not the process of the demyelination also involves the visual pathways, even though demyelination of the optic chiasm has been described in a single study (5). Since there is neither neuro-imaging nor autopsy evidence in this series, this pathogenetic mechanism cannot be firmly taken as the cause of the observed VEP abnormality. Nevertheless, a hypothesis can be put forward that morphological changes affecting the visual tract start early in some patients, and may differ from one patient to another, depending on some factors yet to be established. Pathological studies with special emphasis on the visual pathways on both humans and in experimental models are needed to help localize and characterize these changes.
The presence of the parasite in CSF was associated with neither papilloedema nor prolongation of P100 latency. This lack of association is in accordance with the fact that trypanosomes are not always found in CSF, despite the severity of the clinical manifestations.
To conclude the most frequent neuro-ophthalmologic abnormalities in neurologically affected patients with HAT observed in this study were likely to be associated with increased intracranial pressure and included papilloedema and cranial nerve palsies, most often the abducens nerve. VEPs were abnormal in some patients despite apparently normal clinical examination. P100 latency was prolonged while the N75/P100 amplitude remained normal. VEP is a useful tool to detect subclinical impairment of the visual pathways in patients with HAT and raises the question regarding the underlying neurologic damage in this disorder.
| ACKNOWLEDGEMENTS |
| We wish to thank Drs Mputu Anne-Marie and Nyamabo Louis for their assistance. We are also grateful to Professors Kayembe David and Kayembe Tharcisse, both at the Kinshasa University Hospital, for allowing the study to be conducted in their departments. This study was supported by Norwegian Agency for Development Co-operation and the University of Bergen. |
Table 1
| Manifestations |
|
|
N (%) |
Trypanosome in CSF (+) |
Trypanosome in CSF (-) |
CSF lymphocytes (<5ml) |
CSF lymphocytes (5-20ml) |
CSF lymphocytes (>20/ml) |
CSF protein (45-100 mg%) |
CSF protein(>100 mg) |
| Papilloedema |
|
|
19 (17) |
12 |
7 |
0 |
0 |
19 |
11 |
8 |
| Cranial nerve palsy |
|
|
10 (9) |
9 |
1 |
0 |
1 |
9 |
7 |
3 |
| |
-isolate |
|
8 (7) |
7 |
1 |
0 |
1 |
7 |
6 |
2 |
| |
|
VI |
5 (4) |
4 |
1 |
0 |
0 |
5 |
3 |
2 |
| |
|
VII |
3 (3) |
3 |
0 |
0 |
1 |
2 |
3 |
0 |
| |
-multiple |
|
2 (2) |
2 |
0 |
0 |
0 |
2 |
1 |
1 |
| |
|
VI+VII |
1 (1) |
1 |
0 |
0 |
0 |
1 |
0 |
1 |
| |
|
III+IV+VI |
1 (1) |
1 |
0 |
0 |
0 |
1 |
1 |
0 |
| Visual field defects |
|
|
5 (4) |
2 |
3 |
0 |
1 |
4 |
5 |
0 |
| Cortical blindness |
|
|
2 (2) |
2 |
0 |
0 |
1 |
1 |
2 |
0 |
| Nystagmus |
|
|
1 (1) |
1 |
0 |
0 |
0 |
1 |
1 |
0 |
Number and percent of patients with different types of neuro-ophthalmologic manifestations in 114 neurologically affected patients with HAT. A total of 32 patients (28%) had any neuro-ophthalmological disturbances.
Table 2
| |
Controls |
HAT |
95% C.I. |
| Number of participants |
76 |
110 |
|
| Mean P100 latency ± SD (ms) |
100.7 ± 4.9 |
102.8 ± 9.6 |
-0.3-4.4 |
| Mean amplitude ± SD (uV) |
14.8 ± 5 |
13.4 ± 4.8 |
-2.8-0.07 |
VEP results in neurologically affected patients with HAT compared to controls.
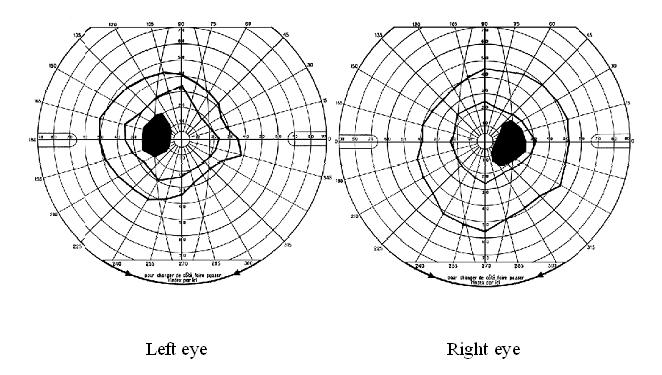
Figure 1
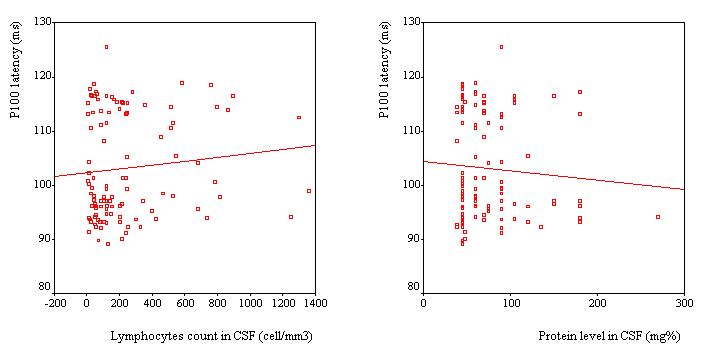
Figure 2
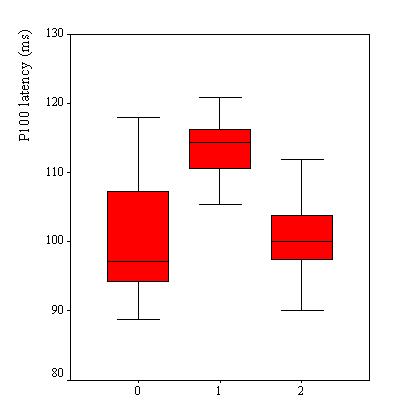
Figure 3
REFERENCES
- BEDAT-MILLET AL, CHARPENTIER S, MONGE-STRAUSS MF, WOIMANT F. Forme psychiatrique de la trypanosomiase humaine africaine: illustration des difficultés diagnostiques et apport de l’imagerie par résonance magnétique. Rev Neurol (Paris) 2000; 156: 505-509.
- BILENGUE CM, MESO VK, LOUIS FJ, LUCAS P. Trypanosomiase humaine africaine en the milieu urbain: l’ exemple de Kinshasa, République Démocratique du Congo, en 1998 et 1999. Med Trop 2001; 61: 445-448.
- BUISSONNIERE RF, DE BOISSIEU D, TELL G, BURSZTYN J, BELLIOT P, PONSOT G. Uvéo-méningite révélant une trypanosomiase ouest africaine chez une fille de 12 ans. Arch Fr Pediatr 1989; 46: 517-519.
- DUMAS M, BOUTEILLE B. Trypanosomiase humaine africaine. C R Seances Soc Biol Fil 1996;190: 395-408.
- DUTERTRE J. Contribution à l’etude de l’encéphalite de la maladie du sommeil. Intérêt de la pneumo-encéphalographie dans les correlations bio-anatomo-cliniques. Thèse Faculté de Médecine de Bordeaux. Accessed on June 20, 2003 at http://perso.wanado.fr/jdtr/these55.htm,
- EKWANZALA M, PÉPIN J, KHONDE N, MOLISHO S, BRUNEEL H, DE WALS P. In the heart of darkness: sleeping sickness in Zaire. Lancet 1996; 348: 1427-1430.
- ENANGA B, BURCHMORE RJ, STEWART ML, BARRETT MP. Sleeping sickness and the brain. Cell Mol Life Sci 2002; 59: 845-848.
- FALSINI B, TAMBURRELLI C, PORCIATTI V, ANILE C, PORRELLO G, MANGIOLA N. Pattern electroretinograms and visual evoked potentials in idiopathic intracranial hypertension. Ophthalmologica 1992; 205: 194-203.
- KEITA M, BOUTEILLE B, ENANGA B, VALLAT JM, DUMAS M. Trypanosoma brucei brucei: a long-term model of human African trypanosomiasis in mice, meningo-encephalitis, astrocytosis, and neurological disorders. Exp Parasitol 1997; 85: 183-192.
- KRISTENSSON K, BENTIVOGLIO M. Pathology of African trypanosomiasis. In: Dumas M, Bouteille B, Buguet A, editors. Progress in Human African Trypanosomiasis, Sleeping Sickness. Paris: Springer; 1999. p. 157-181.
- MOLYNEUX DH. Patterns of change in vector-borne diseases. Ann Trop Med Parasitol 1997; 91: 827-839.
- MULENGA C, MHLANGA JD, KRISTENSSON K, ROBERTSON B. Trypanosoma brucei brucei crosses the blood-brain barrier while tight junction proteins are preserved in a rat chronic disease model. Neuropathol Appl Neurobiol 2001; 27: 77-85.
- POISSON M, BLEIBEL JM, REGNIER A, MASHALY R, LE BIGOT P, DANIS M, et al. Formes pseudo-tumorales de la trypanosomiase africaine à Trypanosoma gambiense. Etude clinique et tomodensitometrique. Sem Hop 1980; 56: 1979-1982.
- RIZZO PA, PIERELLI F, POZZESSERE G, SANCESARIO G, BOATTA M, SANTARCANGELO G. Pattern visual evoked potentials in pseudotumor cerebri. A longitudinal study. Acta Neurol Belg 1984; 84: 57-63.
- SORENSEN PS, TROJABORG W, GJERRIS F, KROGSAA B. Visual evoked potentials in pseudotumor cerebri. Arch Neurol 1985; 42: 150-153.
16. STICH A, ABEL PM, KRISHNA S. Human African trypanosomiasis. BMJ 2002; 325: 203-206.
- VAN NIEUWENHOVE S, BETU-KU-MESU VK, DIABAKANA PM, DECLERCQ J, BILENGE CM. Sleeping sickness resurgence in the DRC: the past decade. Trop Med Int Health 2001; 6: 335-341.
- VERPLANCK M, KAUFMAN DI, PARSONS T, YEDAVALLY S, KOKINAKIS D. Electrophysiology versus psychophysics in the detection of visual loss in pseudotumor cerebri. Neurology 1988; 38: 1789-1792.
- WHO. African trypanosomiasis. http://www.who.int/emc/diseases/tryp/index.html 2001 (accessed on June 20, 2003)
- ZOLA JM, WASSOUMBOU-LOUBIENGA S, GOMA GC, MOUANGA-YIDIKA G. Méningo-encéphalite aiguë à Trypanosoma brucei gambiense révélée par un œdème papillaire. Bull Soc Pathol Exot 1994; 87: 312-314.



