ANATOMY / ANATOMIE
VARIANT ANATOMY OF THE ANTERIOR CEREBRAL ARTERY IN ADULT BRAINS
VARIANTES ANATOMIQUES DE L'ARTÈRE CÉRÉBRALE ANTÉRIEURE CHEZ L'ADULTE
- Department of Human Anatomy, School of Medicine, University of Nairobi, Kenya
ABSTRACT
Background
Aneurysms of the anterior cerebral and anterior communicating arteries are common and their microvascular surgical management requires sound knowledge of the normal and variant vascular anatomy.
Objective
The purpose of this study was to evaluate variations of the anterior cerebral and anterior communicating arteries.
Methods: Thirty six cadaveric brains (72 hemispheres) were studied by gross dissection for the pattern of arterial blood supply.
Results
The anterior cerebral artery (ACA) was observed to originate from the ipsilateral internal carotid artery (ICA) in all the cases studied. The most common type of termination of the ACA was bifurcation into pericallosal (PerA) and callosomarginal (CMA) arteries with the PerA-CMA junction being supracallosal (60%), infracallosal (27%) or precallosal (5%). Unique variations observed include an accessory ACA from the ACoA, ‘bihemispheric pericallosal arteries’, intertwining course of the A2 segments of the ACAs and crossing branches from 1 hemisphere to another. Variations of the ACoA were also observed including fenestration (26%) and duplication (13%).
Conclusions
The majority of ACA bifurcations, in the current study, were supracallosal suggesting the need for exploration of the interhemispheric fissure during surgical corrections of distal ACA aneurysms. Further, the incidence of the callosomarginal artery in this series appears to be at variance with other studies highlighting the need to standardize the definition of the artery. Anterior communicating artery fenestration was the most common variation raising concern as this has been shown to compromise collateral flow and predispose to aneurysm formation.
Key Words: Anterior Cerebral artery, Anterior Communicating Artery, Kenya, Variations
RESUME
Préambule
Les anevrismes de l’artére cérébrale antérieure et des artéres communicantes sont fréquentes, et leur traitement microchirurgical nécessite une bonne connaissance de l’anatomie vasculaire normale ainsi que les variantes anatomiques.
Objectif
Le but de ce travail était d’évaluer l’anatomie variante de l’artère cérébrale antérieure l’artére communicante antérieure dans le Cerveau Adulte.
Methodes
Trente-six cerveaux de cadavres (72 hemisphères) ont été étudiés par microdissection afin d’étudier le statut des vaisseaux de la base du crâne.
Resultats
L’artère cérébrale antérieure (ACA) avait pour origine l’artère carotide interne (ICA) du côté ipsilatéral dans tous les cas étudiés. Le type le plus commun de termination de l’ACA était la bifurcation en artéres péricalleuses (PerA) et calloso-marginales (CMA), la jonction PerA-CMA étant supracalleuse (60%), infracalleuse (27%) ou précalleuse(5%). Les variations observées étaient une ACA accessoire de l’AcoA, des artéres péricalleuses bihémisphériques, un trajet entrelacé des segments A2 de l’ACA et des branches transversant un hémisphère. Des variations de l’AcoA étaient observées avec des fenestrations (26%) et des duplications (13%).
Conclusions
La majorité des bifurcations de l’ACA, dans cette étude, étaient supracalleuse ce qui suggère d’explorer la fissure interhémisphérique pendant les interventions chirurgicales des anévrismes de l’ACA distale. De plus, l’incidence de l’artère callosomarginale semble être variée ce qui souligne la nécessité de standardiser la définition de cette artére. La fenestration de l’artére communicante antérieure était la variation la plus commune.
Mots clés: Artère Cérébrale Antérieure, Artère Communicante Antérieure, Kénya, Variations.
INTRODUCTION
The anterior communicating complex formed by the ACA, ACoA and adjacent branches is a common site for aneurysm formation (1). Microvascular reconstruction procedures used to manage aneurysms require thorough knowledge of the vascular anatomy and variations for planning of surgical strategy (14). Previous reports intimate variations in the vascular anatomy including ACA hypoplasia (1,6), single (Azygos) or triple ACAs (10). The callosomarginal artery may be missing (3) and when present the PerA-CMA junction may be superior, anterior or inferior to the genu of the corpus callosum (4). As data from Africa is scarce, this paper aims to report the variant anatomy of the anterior cerebral artery in Kenyans.
MATERIALS AND METHODS
Thirty six cadaveric brains (72 hemispheres) for routine dissection at the Department of Human Anatomy, University of Nairobi, were used in the study. Any subject with evidence of pathology or trauma of the brain and its supplying vessels that may have affected the topography of the arteries was excluded from the study. Each calvarium was opened using a saw, the dura incised and the brain detached at the spino-medullary junction then carefully lifted out. The arachnoid mater was peeled off to expose the vessels at the base of the brain and their branches identified. The origin, course, termination and variable anatomy of the anterior cerebral and anterior communicating arteries were noted and recorded. The dissection was done with the aid of a hand lens (x10) and representative photographs taken using a digital camera (Sony cybershot 7.2 megapixels). Fenestration of the ACoA was defined as an incomplete separation of the anterior communicating artery while two separate ACoAs were considered duplicated.
RESULTS
Seventy two cases (41 males and 31 females) were available for study. The anterior cerebral artery was the smaller terminal branch of the internal carotid artery and was joined to its fellow ACA by an anterior communicating artery in all the cases. In 66 hemispheres (92%) the A2 segment coursed round the genu of the corpus callosum and terminated as the pericallosal and callosomarginal arteries. Most of the PerA-CMA junctions were supracallosal (60%) while others were infracallosal (27%) or precallosal (5%). In 6 cases (8%) the callosomarginal artery was absent and there was thus no PerA-CMA junction.
An accessory ACA was documented in one brain (Fig. 1). It was given off from the anterior communicating artery communicating artery and accompanied the right and left ACAs around the genu of the corpus callosum. This vessel terminated midway above the body of the corpus callosum by giving branches to both hemispheres.
Early termination of the pericallosal artery was observed in four hemispheres (6%). In these cases, the left pericallosal artery ended in the cingulate sulcus at the level of the genu of the corpus callosum and a collateral branch of the right pericallosal artery vascularised the posterior territory of both hemispheres. This “bihemispheric pericallosal artery” originated at the level of the genu of the corpus callosum and ran backward in the midline within the callosal cistern (Fig 2).
In one case the A2 segments of both ACAs had an intertwining course. Following communication at the ACoA, the right anterior cerebral artery crossed the midline superior to the left ACA and then traversed forwards along the orbital surface. It then recrossed to its own side and had a standard subsequent course (Fig 3).
The ACoA had a variable pattern in 14 hemispheres (40%). Fenestration was observed at an incidence of 26% (Fig 4) while that of complete duplication of the ACoA was 14% (Fig 5).
DISCUSSION
In the present study, a normal terminal ACA bifurcation was recorded in 66 (92%) cases. The callosomarginal artery (CMA) was absent in 8% of the hemispheres. Previous studies have reported disparate rates for the absence of the callosomarginal artery (Table 1), raising questions as to its value as a landmark in the nomenclature of the distal ACA. This difference in reported incidences may be in part due to variable definitions of the callosomarginal artery. According to Rhoton (9), PerA is the primary extension of the ACA beyond ACoA and the CMA is its largest cortical branch. For other authors, CMA comes into existence after its bifurcation point with the PerA (3,8,10,12). In the current study, CMA as the artery originating from the distal ACA, coursing in the cingulate sulcus, and producing cortical branches.
The pericallosal-callosomarginal junction (perA-CMA) in the current study was above, below or in front of the genu of the corpus callosum in 60%, 28%, and 4% of the cases respectively. Most aneurysms of the distal ACA arise at the perA-CMA junction (4,13). This anatomical detail may dictate exploration of the interhemispheric fissure above the corpus callosum for aneurysms of distal ACA in a significant proportion of brains.
Four (6%) of 62 hemispheres had a “bihemispheric pericallosal artery” which ran backward within the callosal cistern supplying both hemispheres. The data by Ture denotes a much higher incidence of 13.3% (12). In one case a third ACA was observed arising from the ACoA and accompanying the two normal ACAs around the genu of the corpus callosum. Acessory ACAs are rare in literature.
Bihemispheric pericallosal and accessory anterior cerebral arteries may be explained by the embryological development of the cerebral arteries. Padget observed an embryonic median artery of the corpus callosum which was a branch of the ACoA directed toward the commissural plate (7). Persistence of the median artery of the corpus callosum into adulthood forms an accessory ACA. However, if one of the two A2 segments is underdeveloped, its territory may be vascularized either by the median artery of the corpus callosum or by the contralateral pericallosal arteries (7). This variation corresponds to the bihemispheric pericallosal artery observed in the current study.
The observed incidence of duplication of the ACoA (14%) in the present study is much lower than reported in literature (Table 2). The presence of variations in the ACoA may also be explained by the embryological development of the ACAs. The ACoA has not yet formed in the 21 mm stage embryo. It is a single large canal in embryos of 23mm, and is large and plexiform in the 24mm stage embryo (7). Incomplete fusion of this plexiform anastomosis may lead to a fenestration or a doubling or tripling of the ACoA (2,7). Gomez et al reported mean diameter values of 1.8± 0.1 mm in the case of a single trunk and of 1.1±0.1 mm in the case of a double trunk (2). Thus, in patients with a double trunk, the mean ACoA resistance could be slightly higher than that of patients with a single trunk (2). It follows that collateral flow would probably be better in individuals with single than double ACoAs. Further, Matsumura and Nojiri (5) reported a high incidence of coexisting fenestration and aneurysms of the ACoA and suggested that congenital factors may play a role in the pathogenesis of cerebral aneurysm.
CONCLUSION
Majority of CMA terminations were supracallosal. ACA were either duplicated or fenetrated in a significant proportion of brains. These anatomical features may form important considerations in the pathogenesis and surgical approach to ACA aneurysms.
Table 1: Table showing the incidence of absence of callosomarginal artery
| Author |
Country |
Prevalence |
| Lemos 1984 |
Portugal |
6.4% |
| Lemos 1984 |
Portugal |
17% |
| Kakou 2000 |
Turkey |
60% |
| Current study |
Kenya |
8% |
Table 2: Table showing the incidence of duplicated ACoA
| Author |
Country |
Duplicated ACoA |
| Perlmutter 1978 |
USA |
30% |
| Hillen 1986 |
USA |
33% |
| De Almeida 1934 |
Spain |
18% |
| Fisher 1965 |
USA |
33% |
| Crowell 1977 |
Unspecified |
33.3% |
| Gomes 1986 |
Spain |
43.3% |
| Current study |
Kenya |
14% |
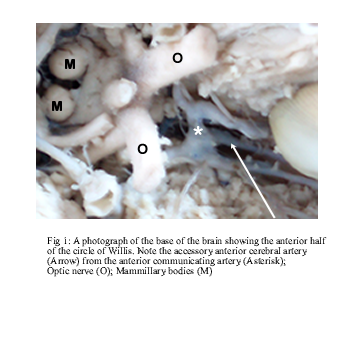
Figure 1
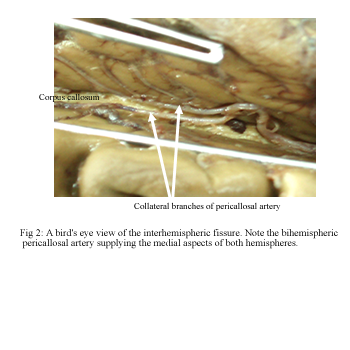
Figure 2
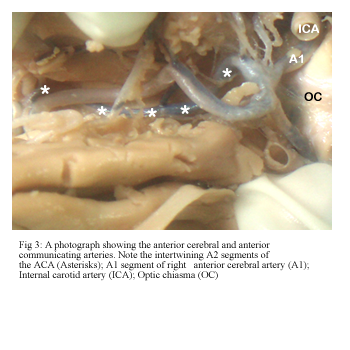
Figure 3
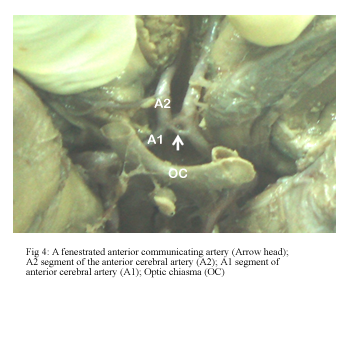
Figure 4
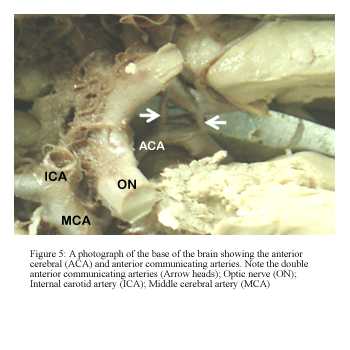
Figure 5
REFERENCES
- ARMAND JP, DOUSSET V, VIARD B, HUOT P, CHEHAB Z, DOS SANTOS E, BERGE J, CAILLE JM. Agenesis of the internal carotid artery associated with an aneurysm of the anterior communicating artery. J Neuroradiol. 1996;23(3):164-7.
- GOMES FB, DUJOVNY M, UMANSKY F, BERMAN SK, DIAZ FG, AUSMAN JI, MIRCHANDANI HG, RAY WJ. Microanatomy of the anterior cerebral artery. Surg. Neurol. 1986;26(2):129-41.
- KAKOU M, DESTRIEUX C, VELUT S. Microanatomy of the pericallosal arterial complex. J. Neurosurg. 2000;93:667-675.
- KAWASHIMA M, MATSUSHIMA T, SASAKI T. Surgical strategy for distal anterior cerebral artery aneurysms: Microsurgical anatomy. J. Neurosurg. 2003;99(3):517-25.
- MATSUMURA M, NOJIRI K. Ruptured anterior communicating artery aneurysms associated with fenestration of the anterior cerebral artery. Surg. Neurol. 1984;22:371-376.
- NAKAMURA H, YAMADA H, NAGAO T, FUJITA K, TAMAKI N. A case of hypoplasia of the left internal carotid manifested as convulsion attack. No Shinkei. Geka. 1993;21(9):843-8.
- PADGET DH. The development of the cranial arteries in the human embryo. Contribution to embryology. Carneg. Instit. 1948;32:205-261.
- PERLMUTTER D, RHOTON AL Jr: Microsurgical anatomy of the distal anterior cerebral artery. J. Neurosurg. 1978;49:204-228.
- RHOTON AL Jr. The supratentorial arteries. Neurosurg. 2002;51:53-120.
- STEFANI MA, SCHNEIDER FL, MARRONE AC, SEVERINO AG, JACKOWSKI AP, WALLACE MC. Anatomic variations of the anterior cerebral cortical branches. Clin. Anat. 2000;13(4):231-6.
- TAO X, YU XJ, BHATTARAI B, LI TH, JIN H, WEI GW, MING JS, REN W, JIONG C. Microsurgical anatomy of the anterior communicating artery complex in adult Chinese heads. Surg. Neurol. 2006;65(2):155-61.
- TURE U, YARSAGIL MG, KRISCHT AF. Arteries of the corpus callosum: a microsurgical anatomic study. Neurosurg. 1996;39:1075-85.
- UGUR CH, GOKMEN PD, ESMER AF, COMERT A, BODABASI A, TEKDEMIR I, ALAITTIN DVM, KANPOLAT Y. A Neurosurgical view of anatomical variations of the distal anterior cerebral artery. J. Neurosurg. 2006;104:278-284.
- YOKOH A, AUSMAN JI, DUJOVNY M, DIAZ FG, BERMAN SK, SANDERS J, MIRCHANDANI HG. Anterior cerebral artery reconstruction. Neurosurg. 1986;19(1):26-35.





