
|
|
|
ORIGINAL PAPERS / ARTICLES ORIGINAUX
HAEMORRHAGIC STROKE IN GABON (Libreville): PROGNOSTIC FACTORS
ACCIDENTS VASCULAIRES CEREBRAUX HEMORRAGIQUES au GABON (Libreville) : FACTEURS PRONOSTIQUES
Summary Hemorrhagic stroke (HS) contributes to 10% to 20% of strokes annually. Hemorrhagic stroke is associated with severe morbidity and high mortality. Objective: to study the hospital frequency and the factors associated with mortality and survival of hemorrhagic stroke (HS) patients at the CHUL in 2022 Patients and methods: This was a retrospective study with descriptive and analytical aims, spanning from 1 January 2018 to 31 December 2022. The study population consisted of HS patients hospitalized in the CHUL neurology department during the study period. Results: We included 505 patients with HS. The majority were male, with a sex ratio of 1.6. The mean age of our population was 55,2 ± 11,8 years. The in-hospital stroke rate was 16.1%. Overall mortality was 23.8%. It was associated with previous stroke, Glasgow and ICH scores at admission, the occurrence of inhalation pneumonitis, and the length of hospitalization. Conversely, factors associated with survival were younger age and absence of stroke. Conclusion: HS is a pathology responsible for high mortality. This study evaluated hospital frequency, mortality and factors associated with mortality and survival. Keywords: Hemorrhagic stroke, Frequency, Associated factors, Mortality, CHUL survival.
Résumé Les accidents vasculaires cérébraux hémorragiques (AVCh) représentent 10 à 20 % de l’ensemble des AVC chaque année. L’AVCh est associé à une morbidité grave et à une mortalité élevée. Objectif : étudier la fréquence hospitalière et les facteurs associés à la mortalité et à la survie des patients ayant subi un AVCh au CHUL en 2022. Patients et méthodes : Il s’agissait d’une étude rétrospective à visée descriptive et analytique, s’étendant du 1er janvier 2018 au 31 décembre 2022. La population étudiée était constituée des patients ayant subi un AVCh et hospitalisés au service de neurologie du CHUL durant la période d’étude. Résultats : On était inclus dans l’étude, 505 patients atteints. La majorité était de sexe masculin, avec un sex-ratio de 1,6. L’âge moyen de notre population était de 55,2 ± 11,8 ans. La fréquence hospitalière des AVCh était de 16,1 %. La mortalité globale était de 23,8 %. Elle était associée aux antécédents d’AVC, aux scores de Glasgow et d’Hemphil à l’admission, à la survenue d’une pneumopathie d’inhalation et à la durée d’hospitalisation. A l’inverse, les facteurs associés à la survie étaient le jeune âge et l’absence d’antécédent d’AVC.
Conclusion : L’AVCh est une pathologie responsable d’une mortalité élevée. Cette étude a permis d’évaluer la fréquence hospitalière, la mortalité et les facteurs associés à la mortalité et à la survie.
Mots-clés : AVC hémorragique, Fréquence, Facteurs associés, Mortalité, Survie CHUL.
Introduction Stroke is a global public health issue. It remains the third-largest killer in industrialized countries and the second-largest killer ahead of infectious diseases in developing countries [18,44]. It is the leading cause of non-traumatic acquired disability in adults [37]. Strokes are subdivided into Ischaemic Stroke (IS) in 85% and Haemorrhagic Stroke (HS) in 15-20% [38]. A stroke is defined as an irruption of blood caused by the rupture of an intracranial pathological blood vessel either in the cerebral parenchymal (intra parenchymal haemorrhage) or in the subarachnoid spaces (subarachnoid haemorrhage) [11]. The aetiologies of haemorrhagic strokes are multiple such as Arterial Hypertension (AHT), considered as the most important cardiovascular risk factor to which is attributed approximately 60 to 75% of intracranial haemorrhages [5,20]. Over the last few decades, the incidence and mortality of haemorrhagic stroke in Europe, America and Asia have not changed significantly. The total incidence based on a meta-analysis performed by Wang et al.in 2022 on 52 included studies was 29.9 per 100,000 person-years. China had the highest incidence with 131 per 100,000 person-years, followed by Greece and Japan. In Sub-Saharan Africa (SSA), data on haemorrhagic stroke is lacking. Few studies are described in the literature. Studies in Uganda, ivory coast, and Senegal reported hospital frequencies of 25.1%, 27.3% and 36.6% respectively [52,4,1]. HS is associated with severe morbidity and high mortality [48]. Diagnosis and early treatment are essential given the usually rapid expansion of brain haemorrhage causing neurological deterioration. The mortality of HS is higher during the acute phase and can be estimated at 50% at one month [50]. The main death risk factors have been described in the literature and include age, clinical severity, volume of haemorrhage, presence of intraventricular haemorrhage [16]. If the prognosis of ischaemic stroke (IS) has been improved in recent years with the arrival of stroke units and the introduction of thrombolysis, that of HS has not changed much. The prognosis is even more severe in our African context, where human, material and financial resources are scarce. In Gabon stroke is the main reason for hospitalization in the neurology department [31], but no studies on the frequency of haemorrhagic stroke and factors associated with mortality and survival have been conducted. Methodology The study was conducted in the neurology department of the University Hospital of Libreville (CHUL). This was a retrospective study with descriptive and analytical claims. It covered the period from 1 January 2018 to 31 December 2022. The study population consisted of HS patients hospitalized in the neurology department of the CHUL during the study period. The outcome was defined by mortality corresponding to the patient’s death at 1 month, 3 months, 6 months, 1 year, 3 years and 5 years. A systematic and comprehensive recruitment of all patients who met the inclusion criteria was conducted. Data was collected by means of a literature review. A standardized survey form was drawn up. It included socio-demographic data, cardiovascular risk factors, clinical data at admission, complications during hospitalization, haemorrhagic stroke topography, length of hospital stay, and the evolution of patients’ health. After collecting data on their characteristics, patients alive on the date of discharge were contacted to provide information on whether they were alive or deceased, by means of a verbal autopsy. Data analysis was carried out with R 4.2.1 software. Quantitative descriptive variables were expressed on average with the standard deviation when the distribution was normal (appreciated by the Shapiro Test). The qualitative ones were expressed in percentages. The survival curve was plotted using the Kaplan Meier Method to describe the odds of survival at time t (St). The survival curves were compared by the Logrank Test. The exploratory study of factors associated with mortality was structured in bivariate and multivariate analysis. In bivariate analysis, the uncorrected Pearson Chi-2 test (theoretical numbers > to 5) and the exact Fisher test for small numbers (theoretical numbers less than 5) were used to compare proportions. In multivariate analysis, a descending step-by-step logistic regression was used to identify variables associated with mortality. The initial model had all independent variables that had a p-value of less than 20% in bivariate analysis, age and sex considered as forced variables. Adjusted with their 95% confidence intervals, Odds Ratios (OR) were generated to quantify the strength of associations. The adequacy of the model was verified with the Hosmer-Lemeshow Test. The difference in comparisons was considered significant for p < 0.05 values. Results There were 3337 patients hospitalized in the Neurology Department of the CHUL during the study period. Among them, 2808 (84.1%) had a stroke of which 538 were haemorrhagic strokes. During the inclusion procedure of haemorrhagic stroke cases, 32 patients were excluded from the data analysis because of incomplete records. The completeness rate of haemorrhagic stroke during the study period was 93.9% as shown in the flowchart (Figure 1). The hospital frequency of haemorrhagic stroke was 16.1% considering all admissions to the service. The average age was 55.2±11.8 years with the extremes of 23 and 94 years. The sex ratio was 1.6 in favour of men i.e., 311 (61.6%). In most cases, patients were self-employed (37.2%). While 24.2% were unemployed. More than half of the patients lived in couples (62%). Those with a secondary or higher level of study accounted for 46.5% and 26.1%, respectively. One in two patients had public health insurance (87.6%). Table I shows the distribution of patients by socio-demographic characteristics. Cardiovascular risk factors were dominated by high blood pressure (96.4%) and alcohol consumption (56.2%). Table II presents the cardiovascular risk factors. The mean stroke severity score at admission was 9.9±4.2 with a mean Glasgow score of 14.1±1.8. Mean systolic and diastolic arterial pressures were 170.2±77.1 mmHg and 106.5±15.7 mm Hg, respectively. Table III presents data on patients’ clinical parameters at admission. HS were mostly deep in 83.2% of cases. The Hemphill score ranged from 0 to 3. It was zero (0) in 43.6% of cases and 2 for 21.2% of patients. Table IV gives a breakdown of patients by haemorrhagic stroke location. One in two patients had stayed in the neurology department between 8 and 14 days. Complications arising during hospitalization were dominated by undetermined infections (16.8%), followed by seizures (2.8%), pneumonia (2.6%) and urinary tract infections (0.8%). The outcome of the 428 survivors was assessed after discharge from hospital. One hundred and twenty-one (121) patients were lost to follow-up throughout the study period. Considering alive the patients lost to follow-up, mortality was 4.4% at 1 year. Cumulative mortality was 10% over the 5-year period as described in Table V. Conversely, considering all the patients lost to follow-up as deceased, the mortality at 5 years was 38.3%. Considering the patients who died during hospitalization (77) and the 43 who died during the follow-up, 120 patients died from haemorrhagic stroke in the study. Thus, the estimated mortality was 23.8%. Moreover, considering the 121 patients lost to follow-up as deceased, the mortality from haemorrhagic stroke could reach 47.72%. Patients’ survival likelihood was 90,1% [IC95% :87,5% – 92,7%] at 1 month, 86,3% [IC95% :83,4% – 89,4%] at 3 months, 83,1% [IC95% :79,9% – 86,5%] at 6 months, 82,1% [IC95% :78,8% – 85,5%] at 1 year , 79,7% [IC95% :76,2% – 83,3%] at three years and from 79.7% [IC95% : 76.2% – 83.3%]at five years. As shown in Figure 2. The survival likelihood of patients with previous stroke is significantly lower compared to patients who had their first stroke (p <0.0001). At 5 years, the survival probabilities were 45% [IC95%: 29.1% – 69.6%] in patients who had reoccurrences and 81.1%[IC95%: 77.6% – 84.7%] in patients who had their first stroke. As shown in Figure 3. After adjustment on patients’ characteristics, haemorrhagic stroke mortality was significantly associated with history of previous stroke, Glasgow score at admission, the occurrence of pneumonia during hospitalization and Hemphill score. With identical characteristics, patients with a history of previous stroke were 4.5 times more likely to die (p=0.003). Also, patients with a Glasgow score of less than 13 at admission were 3.1 times more likely to die compared to patients who had a normal state of consciousness at admission. Similarly, when the patient’s stay was complicated by pneumonia, the risk of death was 4.7 times higher. With a Hemphill score of 3, the risk of death was 44 times higher compared to patients who had a zero Hemphill score. This data are presented in Table VI. Discussion Haemorrhagic stroke (HS) accounts for 10-20% of all strokes per year [49]. Our prevalence was within this range. It is slightly higher than that found by Amal and al. (2020) in Tunisia, i.e13.5% [8]. However, higher prevalence was reported by Anayo and al. (2015) in Togo[6] and by Mapoure and al. (2014) in Cameroon, respectively 23.56% and 56% [36]. Boubayi and al. (2020) [10] in Congo also observed a high prevalence of 56.3%. The frequency of our study seems lower than that found in several African studies as it is calculated on a high number of admissions. Higher prevalence is found in the Asian population [21,55]. They are higher than the 15% observed in most developed countries in North America and Western Europe [21,39]. High rates of HS in the Asian population are due to a high prevalence of arterial hypertension or poor control of hypertension in this population [21]. Hispanics and blacks also have a higher relative risk of 2.3 and 3.5 than the white population 32]. We noted a male predominance in our study. Bonkougou et al. (2014)in Burkina Faso [9] and Soro and al. inivory coast [47] also found a male predominance or gender ratio of 1.80 and 1.7, respectively. Corso and al. (2004) in Italy made the same observation [17].However, some studies have found a female predominance. Raveloson and al. (2008) in Madagascar, reported a sex ratio of 0.78 [43]; Anayo and al. (2017) inTogo had a sex ratio of 0.93 [6]. Male predominance is thought to be due to the effect of testosterone, which increases arterial stiffness, making the male population more at vascular risk than female subjects [42]. Also in Africa, social activity relies more on men who may be more exposed to stress. The average age of our study is close to that found by Abdourahaman and al.in Senegal in 2018 [2] and Atrous and al. in Tunisia in 2020 [8] of 59.2±13 years and 66 years respectively. Age is the most important risk factor for stroke. The effect of aging on micro-vascularization of the brain is well known and includes decreased vascular density, thickening of the basal membrane of the vessels, endothelial dysfunction and increased permeability of the blood-brain barrier [50,13]. The overall mortality of our study is comparable to the results of other hospital studies conducted in Sub-Saharan Africa (SSA). Anayo K and al. [6] in Togo and Akani andal.in Ivory Coast (2017) found a HS mortality of 19.85% and 31.1% respectively [4]. With Garbusinski and al. (2016) in Gambia, the mortality rate was 41% in the first 30 days, and the risk of death after a HS was multiplied by 2.5 [25]. Das and al. in Uganda in 2012 noted 38% of mortality for HS compared to 27% for IS. They reported up to 50% mortality from the second week [19]. Globally, the case-fatality rate for HS is about 40% at 1 month, 54% at 1 year and 70% within 5 years of onset. Only 12-39% of patients achieve long-term functional autonomy [7,15,27]. In Africa, high mortality in the acute phase may reflect the scarcity of human, technical and pharmacological resources. In many countries, patients are still treated in general medical wards and not in conventional neurology departments or even better, in proper neurovascular units [3,34,45]. But there is also the poor accessibility to hospital care, and late presentation of a severe stroke in the acute phase. Adversely, in developed countries, a significant decrease in case-fatality rate has been observed since 2020 [1,3,45,46]. This decrease is attributed to improvements in intensive care [34]. Early deaths, which usually occur within the first 30 days may be related not only to the volume of the haematoma, intraventricular location, sub-tentorial location, but also to the patients’ comorbidities [5]. This explains why mortality remains high despite optimal care even in developed countries. Long-term post-stroke outcomes in SSA are poorly described, as most studies are conducted in hospitals. However, these studies cannot give an accurate picture of stroke epidemiology because the majority of stroke cases may not be hospitalized [14]. Few studies examine outcomes beyond 30 days after stroke, let alone HS. Walker et al. in 2015 in Tanzania observed 3 years and 5 years mortality of 60% and 71.8%, respectively [51]. These case-fatality rates were linked to the patient’s socio-economic level. The study was conducted in rural areas where agriculture is the main livelihood (prevention of vascular risk factors is often not a priority because of the high cost of medicines). However, lower mortality rates were described by Adoukonou et al.in Benin in 2020, where 3-year and 5-year mortality accounted for 21.5% and 23.5% [3]. It was possibly linked to the presence of a stroke unit. Several mechanisms could explain the high mortality of SSA in the long run. On the one hand, the poor control of cardiovascular risk factors related to the high cost of medicines. On the other hand, the initial severity of the clinical picture and the lack of long-stay facilities for severe strokes (because many complications cannot be managed at home or in primary care available in SSA). Patients with a history of previous stroke were at higher risk of death. Owolabi Lukman and al.in Nigeria in 2013 observed that a second stroke or more increased the risk of stroke-related death at 1 month by 2.6 times [23]. Lekoubou and al.in 2017 in Cameroon demonstrated an excess mortality of 43% after a reoccurrence of stroke. Possible reasons for the excess mortality in the recurrent stroke group included older age, and more severe stroke [33]. Adoukonou and al. in Parakou found that recurrent stroke (30.5%) was one of the leading causes of death. This should encourage a secondary prevention strategy based on compliance for better control of risk factors [3]. A Glasgow score at admission of less than 13 was predictive of death. Unconsciousness was reported as the most recognized prognostic determinant of death in acute stroke and is directly related to the severity of neurological damage [3]. Pneumonia was observed by other authors to increase the risk of death. Kuate-Tegueu and al. [12] in 2012 in Cameroon (p = 0.001), as well as Owolabi Lukman and al. in Nigeria in 2013 observed that pneumonia was associated with a 10% risk of death by stroke [23]. The mortality rate was threefold and hospital stays were prolonged. Moreover, Mapoure and al. in Cameroon found in 2013 that pneumonia was a frequent complication but was not associated with mortality (P=0.41) [36]. They explained that the frequency of pneumonia in our African context, is related to the fact that eating well when sick is a sign that reassures the family and therefore, even before reaching the neurology services, many patients already have inhalation pneumonia due to food ingestion. A significant link was also found between death and Hemphill score with rates of 5%, 12.8%, 66.4% and 81.8% for scores of 0.1.2 and 3. In our study, the higher the score the greater the risk of death. This result is similar to that of Abdouraman and al. [2] in Senegal who reported rates of 13%, 26% and 73% for scores of 0 to 3. Mortality was significantly related to the length of hospital stay. This result is similar to that found by Awono Ateba and al. [7] in 2016 with rates ranging between 30% and 60% for hospital stays of less than 21 days. However, Nyangui and al. in Gabon in 2022 found that the length of hospitalization was a factor rather associated with the poor functional prognosis of patients [28]. This implies that early mobilization at home reduces the risk of death. It can be assumed in both our study and Awono’s study that severe patients spend less time in hospital because of the early onset of death. Survival after stroke is an important indicator in monitoring stroke burden [29,35]. The survival likelihood decreased during our study period. Several studies have observed a decrease in mortality, and therefore an increase in the survival likelihood after stroke [40,30]. However, updated data on survival by country and by stroke subtypes are rare and even more so for HS [52]. A 2014 meta-analysis estimated 1-year survival at 46% and 5-year survival at only 29% [22,53]. This survival likelihood is probably linked to a high proportion of elderly subjects. Indeed, the probability of survival of patients aged 60 years was lower, even in our study. A younger age at the onset of stroke haemorrhage is predictive of survival even after 20 years [24,54] However, the high survival likelihood found in our study may suggest a better secondary prevention of stroke strategy in Gabon. Also, Gabon is one of the few SSA countries to benefit from mandatory health insurance reducing the cost of stroke by 2/3[29]. Strengths of the Study The survey team consisted of the medical staff, supervised by a neurologist, which helped to limit bias. Additionally, the sampling was exhaustive with a high sample size. Thus, our data can be generalized. This is the first study on haemorrhagic stroke conducted in the Neurology Department of the CHUL, the only neurology service of reference in GABON. Conclusion The frequency of haemorrhagic stroke in CHUL neurology department was 16.9%. Mortality at 1 month, 1 year, 3 years and 5 years varied over the study period. It was the highest at 5 years. The associated factors after multivariate analysis were history of previous stroke, Glasgow score at admission below 13, the occurrence of pneumonia, Hemphill score of 3 and short hospitalization. The likelihood of survival decreased during the study period. Age and stroke recurrence were factors related to survival probability. 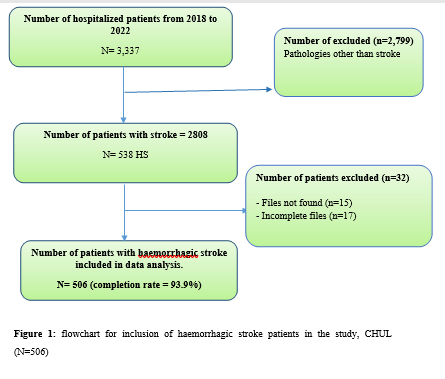 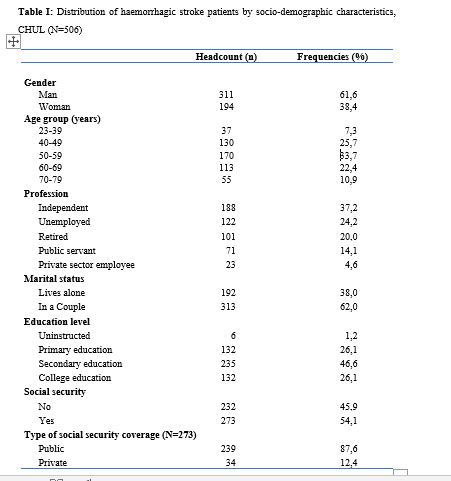 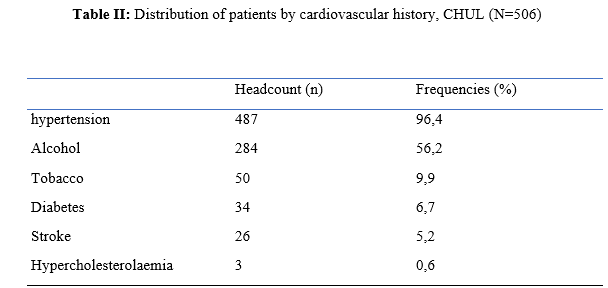 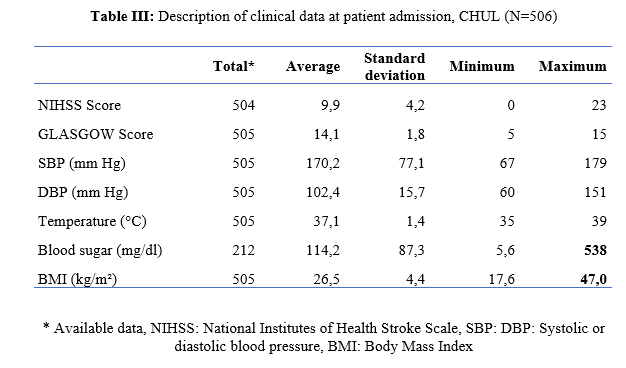 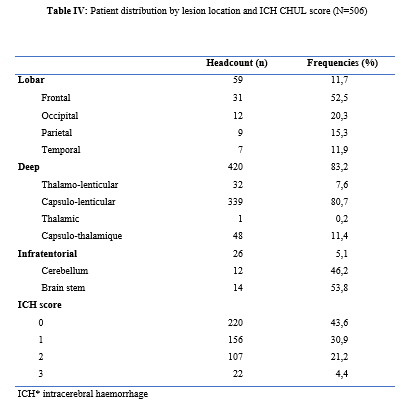 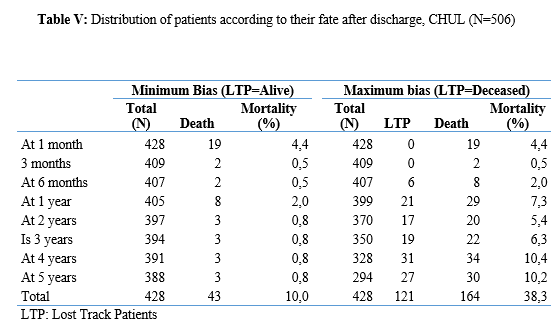 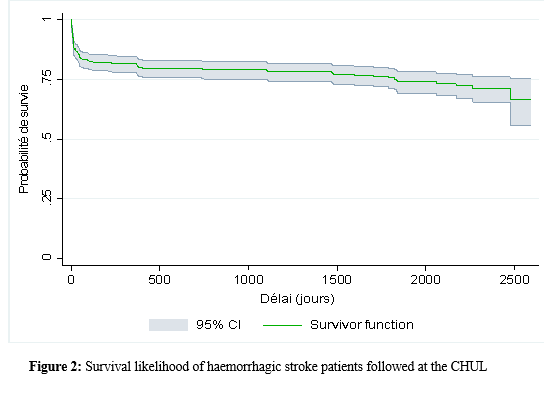 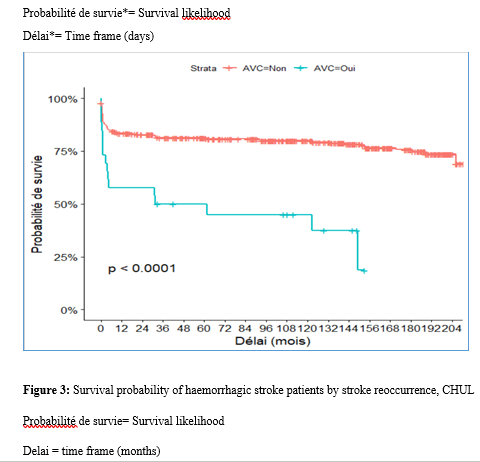 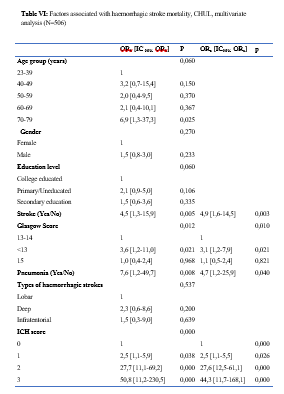 References
|
© 2002-2018 African Journal of Neurological Sciences.
All rights reserved. Terms of use.
Tous droits réservés. Termes d'Utilisation.
ISSN: 1992-2647