
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
ORIGINAL PAPERS / ARTICLES ORIGINAUX
IMMUNOHISTOCHEMICAL ANALYSIS OF PITUITARY ADENOMAS IN A WEST AFRICAN HOSPITAL
ANALYSE IMMUNOHISTOCHIMIQUE DES ADÉNOMES DE L'HYPOPHYSE DANS UN HÔPITAL DE L'AFRIQUE DE L'OUEST
E-Mail Contact - SALAMI Ayodeji :
ayodejisalami@gmail.com
ABSTRACT Purpose Methods Results Conclusion Keywords: Pituitary, Adenoma, Classification, Immunohistochemistry, Endocrine. INTRODUCTION Pituitary adenomas constitute the third most common intracranial neoplasm in adults after gliomas and meningiomas [8,7,1]. Studies from Europe and United States of America have shown a prevalence of 5% to 20% based on cancer registry figures while autopsy and radiological studies have shown a prevalence of 14% and 22.5% respectively[2,9,3, 17,5, 6,24]. Studies done in our environment have indicated a prevalence of 16.8% to 21% [8,7]. Pituitary adenomas are benign tumors but can cause significant morbidity due to pressure effects on neighboring structures, raised intracranial pressure and also due to their functional hormonal effects [13,4,12,15]. The pituitary gland weighs approximately 0.5g but has been called “the conductor of the orchestra” based on the various hormones produced by the anterior part of the gland that controls the activities of many other important endocrine organs in the body [2,17,16,14]. The hormones include prolactin, growth hormone, adrenocorticotropic stimulating hormone, thyroid stimulating hormone, follicle stimulating hormone and luteinizing hormone. Different population of cells produce individual hormones (although rare acidophil stem cells produce Prolactin and growth hormone while many of the gonadotrophs produce both LH and FSH) with each group localized to different parts of the gland [14,22]. Pituitary adenomas can either be functional or silent. The functional adenomas often produce symptoms due to the excess hormone produced by the tumor. Silent adenomas are often macroadenomas and although their sub classification is usually identified by immunohistochemical techniques, they do not show clinical effects. Although many studies have been done on the epidemiology of intracranial tumors in the African setting, a careful search of literature showed no study on the sub classification of pituitary adenomas in our environment. MATERIALS AND METHODS This is a retrospective study of all surgical specimens of pituitary adenomas obtained at the University College Hospital, Ibadan over a twelve year period. The record of the age and gender of the patients were obtained from their histology request cards. The archived slides and paraffin wax blocks were retrieved and the slides were reviewed to ascertain the accuracy of the initial diagnoses and to determine if they satisfied the inclusion criteria for the study. The clinical data, gross appearance and microscopic details at the time of initial diagnoses were also reviewed. Six serial sections cut at 5 microns each were obtained from the archived paraffin blocks for each case. Antigen unmasking with the heat induced epitope retrieval and the pretreatment methods are shown in table 1. Data obtained include age distribution of patients with pituitary adenoma, frequency of the different immunohistochemical groups, sex and age distribution of the different immunohistochemical subgroups. Statistical analysis using Student t-test for comparison of continuous variables and chi-squared test for comparison of discontinuous variables was performed to determine whether there was any association between the various clinical, and immunohistochemical data. The level of statistical significance was set at p ≤ 0.05. RESULTS A total of 47 patients had their samples included in the study. All of the 47 samples had histological diagnosis of pituitary adenoma and also met the study criteria. Thirteen samples were excluded from the study due to inadequate number of sections from the tissue block. Twenty-five (53.2%) of the patients whose samples met the study criteria were females and 22 (46.8%) were males. The ages of the 47 patients with pituitary adenomas in this study ranged from 9 to 76 years with a mean age of 42 years. The overall peak age of diagnosis of pituitary adenomas occurred in the fifth decade of life (figure 1). The peak age for females was in the fourth decade while that of males was in the sixth decade. However this difference was not significant (χ2 = 9.26, degrees of freedom (df) = 7, p = 0.235). Null cell and gonadotroph adenomas were the commonest tumors seen with both constituting thirty four per cent of cases each. Prolactinomas were the next most common while corticotroph and somatotroph adenomas were least common (figure 2 and 3). There was no case of thyrotroph adenoma seen in the study. Expression of more than one hormone was common in the gonadotroph adenomas with both LH and FSH positivity in eleven (68.8%) of the sixteen gonadotrophs. In these neoplasms, FSH staining was usually diffuse, whereas LH staining was often focal (figure 4 and 5). Four gonadotroph adenomas showed exclusive FSH positivity, while one gonadotroph adenoma showed exclusive LH positivity. Growth hormone and prolactin expression was also seen in three cases. One case of corticotroph adenoma also showed focal positivity for TSH. All the adenomas with exception of gonadotroph adenomas occurred more frequently in females (Table 2). Gonadotroph adenomas occurred three times more frequently in males than in females and the difference was statistically significant (χ2 = 7.74, degrees of freedom (df) = 1, p = 0.005). Null cell, corticotroph and gonadotroph adenomas occurred more frequently in the third to sixth decades while growth hormone adenomas were seen more frequently in the second and third decades (Table 3). Prolactinomas had a wide age distribution but had a higher frequency in the second to fourth decade. DISCUSSION According to the 2004 World Health Organization histological classification of endocrine tumors, immunohistochemical assessment of a pituitary adenoma is essential for the optimal management of the patient [13]. Pituitary adenomas were slightly more common in females than in males in this study. This is in keeping with earlier documentation [20]. Although reasons for this are not entirely clear, it has been postulated that the effect of steroidal hormones that are produced in higher quantity in women may play a role. It is also felt that the earlier appearance of clinical symptoms in women may encourage earlier detection [20]. In the present study, null cell adenomas and gonadotroph adenomas were the two most common pituitary adenoma subtypes identified, each accounting for 34% of the cases. Prolactinomas were the third most frequent pituitary adenoma subtype and accounted for 14.9% of the cases. By contrast to our findings, earlier Caucasian studies have reported a predominance of prolactinomas, which accounted for up to 39% of all cases of pituitary adenoma [11,21,10]. However, the frequency of diagnosis of prolactinoma in the histology laboratory has decreased significantly in developed countries because most patients are managed medically using Dopamine agonists such as bromocriptine and do not require surgery [3,10]. Medical treatment of pituitary adenomas might partly account for the lower frequency of prolactinoma in this study. In more recent Caucasian series, it was reported that gonadotroph adenomas and non-secreting adenomas now account for the majority of tumors seen in histology, which is in agreement with our findings [10]. An additional explanation for the high ratio of null cell and gonadotroph adenomas to prolactinomas in the present study may be the occurrence of genetic variations in pituitary adenomas seen in African as compared to Caucasian patients. Growth hormone adenomas are the least common of the adenomas seen in this study. It occurred predominantly in young adults between the ages of 10 to 30yrs. The gigantism often caused by this tumor may account for its earlier detection in the young. The effect of the tumor is mainly mediated through its effect on the liver with the production of IGF-1[13]. The frequency of this tumor in our study differs from Caucasian series where it is the third most common adenoma after prolactinomas and null cell adenomas. This difference may be due to racial differences although Lloyd et al has noted that the use of medical treatment in somatotroph adenomas accounts for reduced frequency in some series [3,13]. Corticotroph adenomas are the third commonest tumors in this series and occurred more frequently in women. The frequency of ACTH adenomas and sex predilection in this study is in agreement with those in Caucasian series [10]. Gonadotroph adenoma occurred three times more frequently in men than in women in this study. The tumor also occurred in the middle to late decades. Most (68.8%) of the gonadotroph adenomas seen in this study showed immunoreactivity for both FSH and LH. Lloyd et al has documented the higher frequency of gonadotrophs in men as was seen in our study [13]. It has further been postulated that the high levels of gonadotroph hormones produced in women during menopausal period, which often occurs during the peak period of occurrence of gonadotroph adenomas, plays a protective role in women[13,20]. Sixty eight per cent of the gonadotroph adenomas showed dual positivity for both FSH and LH. This was followed by exclusive staining by FSH and one that is exclusively LH. These findings are in keeping with Saeger et al’s findings in their study of pituitary adenomas seen in the German registry [2,13,19]. Gonadotroph adenomas which demonstrate positivity for both FSH and LH usually demonstrate stronger and more diffuse staining for FSH, as was observed in this study [13]. Further work however needs to be done to determine the population of cells that stained for the two hormones so as to ascertain whether there is bihormonality of individual tumor cells or whether there are two distinct populations of cells each producing just one of the two hormones in the same tumor. Most of the null cell adenomas seen in this study occurred from the fourth decade onwards and showed a slight female preponderance. Although our study is in agreement with previous work that has shown very rare occurrence of null cell adenomas below the fourth decade, our series shows a slight female preponderance which is in contrast with Caucasian studies which have shown male preponderance [13]. The explanation for this difference may be a reflection of genetic differences and will require further studies. No case of thyrotroph adenoma was seen in this series. Thyrotroph adenomas are the least common of the pituitary adenomas and constitute about one percent of all pituitary adenomas [13,24]. Only one case of plurihormonal adenoma was seen in this study. This tumor showed positive staining for both ACTH and TSH which are both from separate developmental pathways. Plurihormonal adenomas have been defined as tumors expressing more than one hormonal phenotype that cannot be explained by normal cytophysiology or developmental mechanisms [13]. Thus tumors expressing LH and FSH or prolactin, growth hormone and TSH are not included in this group. Plurihormonal adenomas are very rare and show no sex predilection [13]. ACKNOWLEDGEMENTS We wish to thank Dr. Adeolu and Dr. Adeleye of the Dept. of Neurosurgery and Prof. Ogunbiyi, Drs. Oluwasola, Okolo, Ogun, Adeoye and Eze of the Department of Pathology, UCH, Ibadan whose patients and original specimens contributed to our pool. We wish to thank the Breast Cancer Research Laboratory staff in IAMRAT, College of Medicine, University of Ibadan for performing the immunohistochemical procedures. DISCLOSURES The authors declare that they have no conflict of interest with regard to this study. Disclosure of funding: There was no external funding for this work. 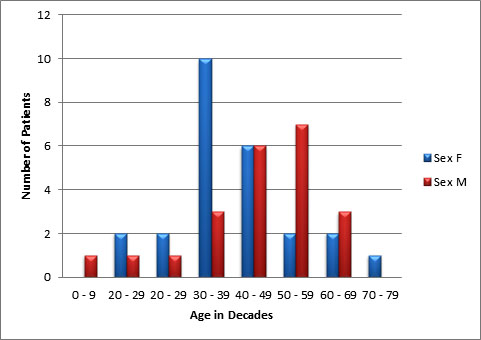 Figure 1 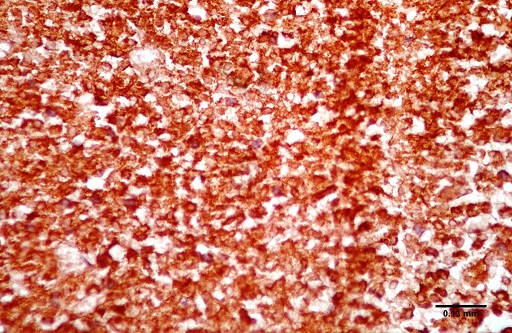 Figure 2 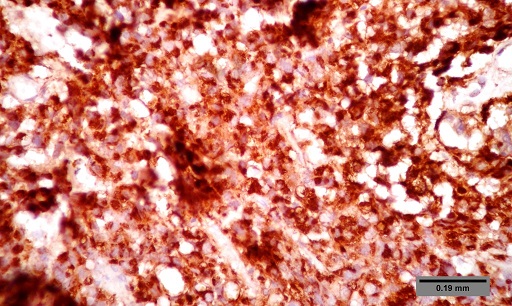 Figure 3 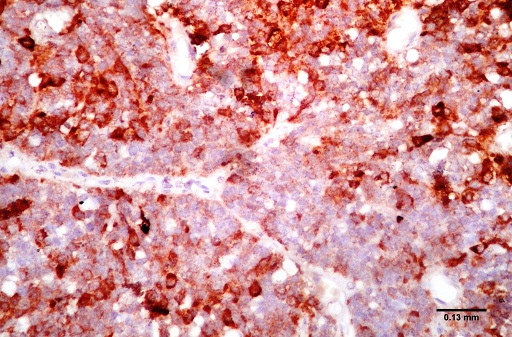 Figure 4 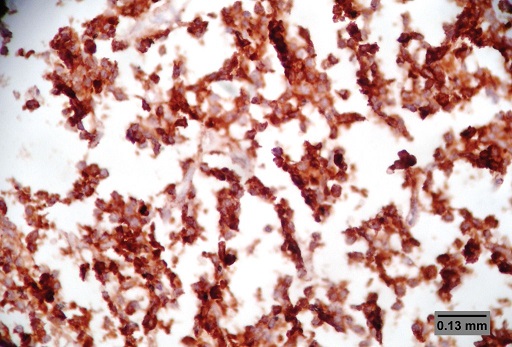 Figure 5 Table 1: Immunohistochemical procedure
LH – Luteinizing hormone, FSH – Follicle Stimulating Hormone, TSH – Thyroid Stimulating Hormone, GH – Growth hormone, ACTH – Adrenocorticotrophic hormone Table 2: Sex distribution of patients with different types of pituitary adenoma
Table 3: Age distribution of patients with different types of pituitary adenoma
REFERENCES
|
© 2002-2018 African Journal of Neurological Sciences.
All rights reserved. Terms of use.
Tous droits réservés. Termes d'Utilisation.
ISSN: 1992-2647